The potential of label-free nonlinear optical molecular microscopy to non-invasively characterize the viability of engineered human tissue constructs
- PMID: 24854093
- PMCID: PMC4106121
- DOI: 10.1016/j.biomaterials.2014.04.080
The potential of label-free nonlinear optical molecular microscopy to non-invasively characterize the viability of engineered human tissue constructs
Abstract
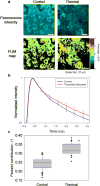
Nonlinear optical molecular imaging and quantitative analytic methods were developed to non-invasively assess the viability of tissue-engineered constructs manufactured from primary human cells. Label-free optical measures of local tissue structure and biochemistry characterized morphologic and functional differences between controls and stressed constructs. Rigorous statistical analysis accounted for variability between human patients. Fluorescence intensity-based spatial assessment and metabolic sensing differentiated controls from thermally-stressed and from metabolically-stressed constructs. Fluorescence lifetime-based sensing differentiated controls from thermally-stressed constructs. Unlike traditional histological (found to be generally reliable, but destructive) and biochemical (non-invasive, but found to be unreliable) tissue analyses, label-free optical assessments had the advantages of being both non-invasive and reliable. Thus, such optical measures could serve as reliable manufacturing release criteria for cell-based tissue-engineered constructs prior to human implantation, thereby addressing a critical regulatory need in regenerative medicine.
Keywords: Fluorescence lifetime imaging microscopy (FLIM); Label-free optical molecular imaging; Multiphoton excitation microscopy; Second-harmonic generation (SHG) imaging; Tissue engineering; Tissue viability.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures






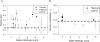
Similar articles
-
Optical Metric Assessed Engineered Tissues Over a Range of Viability States.Tissue Eng Part C Methods. 2019 May;25(5):305-313. doi: 10.1089/ten.TEC.2018.0344. Tissue Eng Part C Methods. 2019. PMID: 30973066 Free PMC article.
-
Noninvasive Optical Assessment of Implanted Engineered Tissues Correlates with Cytokine Secretion.Tissue Eng Part C Methods. 2018 Apr;24(4):214-221. doi: 10.1089/ten.TEC.2017.0516. Tissue Eng Part C Methods. 2018. PMID: 29448894 Free PMC article.
-
Quantitative, Label-Free Evaluation of Tissue-Engineered Skeletal Muscle Through Multiphoton Microscopy.Tissue Eng Part C Methods. 2017 Oct;23(10):616-626. doi: 10.1089/ten.TEC.2017.0284. Epub 2017 Sep 20. Tissue Eng Part C Methods. 2017. PMID: 28810820 Free PMC article.
-
Multiphoton microscopy for blood vessel imaging: new non-invasive tools (Spectral, SHG, FLIM).Clin Hemorheol Microcirc. 2007;37(1-2):77-88. Clin Hemorheol Microcirc. 2007. PMID: 17641398 Review.
-
Taking a deep look: modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine.J R Soc Interface. 2013 Jul 17;10(86):20130263. doi: 10.1098/rsif.2013.0263. Print 2013 Sep 6. J R Soc Interface. 2013. PMID: 23864499 Free PMC article. Review.
Cited by
-
Non-linear optical flow cytometry using a scanned, Bessel beam light-sheet.Sci Rep. 2015 May 29;5:10751. doi: 10.1038/srep10751. Sci Rep. 2015. PMID: 26021750 Free PMC article.
-
Bioengineering tools for probing intracellular events in T lymphocytes.WIREs Mech Dis. 2021 Jul;13(4):e1510. doi: 10.1002/wsbm.1510. Epub 2020 Oct 19. WIREs Mech Dis. 2021. PMID: 33073545 Free PMC article. Review.
-
Optical Metric Assessed Engineered Tissues Over a Range of Viability States.Tissue Eng Part C Methods. 2019 May;25(5):305-313. doi: 10.1089/ten.TEC.2018.0344. Tissue Eng Part C Methods. 2019. PMID: 30973066 Free PMC article.
-
Noninvasive Optical Assessment of Implanted Engineered Tissues Correlates with Cytokine Secretion.Tissue Eng Part C Methods. 2018 Apr;24(4):214-221. doi: 10.1089/ten.TEC.2017.0516. Tissue Eng Part C Methods. 2018. PMID: 29448894 Free PMC article.
-
FRAP, FLIM, and FRET: Detection and analysis of cellular dynamics on a molecular scale using fluorescence microscopy.Mol Reprod Dev. 2015 Jul-Aug;82(7-8):587-604. doi: 10.1002/mrd.22501. Epub 2015 May 25. Mol Reprod Dev. 2015. PMID: 26010322 Free PMC article. Review.
References
-
- Izumi K, Song J, Feinberg SE. Development of a tissue-engineered human oral mucosa: from the bench to the bed side. Cells Tissues Organs. 2004;176:134–152. - PubMed
-
- Hellman KB. Tissue engineering: translating science to product. Ashammakhi N, Reis R, Chiellini F, editors. Topics in Tissue Engineering. E-book. 2008:1–28. Available from URL: http://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol4/
-
- Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1–15. - PubMed
-
- Pitts J, Sloboda R, Dragnev K, Dmitrovsky E, Mycek M-A. Autofluorescence characteristics of immortalized and carcinogen-transformed human bronchial epithelial cells. J Biomed Opt. 2001;6:31–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

