Strong non-classical induction forces in ion-surface interactions: general origin of Hofmeister effects
- PMID: 24854224
- PMCID: PMC7365329
- DOI: 10.1038/srep05047
Strong non-classical induction forces in ion-surface interactions: general origin of Hofmeister effects
Abstract
Hofmeister effects continue to defy all-encompassing theories, and their origin is still a matter of debate. We observed strong Hofmeister effects in Ca2+/Na+ exchange on a permanently charged surface over a wide range of ionic strengths. They could not be attributed to dispersion forces, classical induction forces, ionic size, or hydration effects. We demonstrated that another stronger force was active in the ion-surface interactions, and which would create Hofmeister effects in general. The strength of this force was up to 10(4) times that of the classical induction force, and could be comparable to the Coulomb force. Coulomb, dispersion and hydration effects appeared to be interwined to affect the force. The presence of the observed strong non-classical induction force implied that energies of non-valence electrons of ions/atoms at the interface might be heavily underestimated in current theories, and possibly just those underestimated energies of non-valence electrons determined Hofmeister effects.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

 ) and experimental data (
) and experimental data ( ). KE is
the experimental selectivity coefficient; KC is the calculated selectivity
coefficient based on the classical theory.
). KE is
the experimental selectivity coefficient; KC is the calculated selectivity
coefficient based on the classical theory.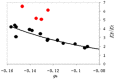

 ) and KE (
) and KE ( ).
).
 ) and KE (
) and KE ( ).
).
 vs.
φ0 (V) under different a0Na/a0Ca
ratios (numbers aside dots are the values of a0Na/a0Ca).
vs.
φ0 (V) under different a0Na/a0Ca
ratios (numbers aside dots are the values of a0Na/a0Ca).
References
-
- Kunz W, Henle J, Ninham B. ‘Zur Lehre von der Wirkung der Salze’(about the science of the effect of salts): Franz Hofmeister's historical papers. Curr. Opin. Colloid Interface Sci. 2004;9:19–37. doi: 10.1016/j.cocis.2004.05.005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

