Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury
- PMID: 24854266
- PMCID: PMC4243351
- DOI: 10.1681/ASN.2013101079
Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury
Abstract
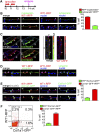
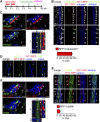
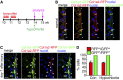
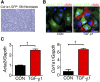
Fibrosis of the peritoneal cavity remains a serious, life-threatening problem in the treatment of kidney failure with peritoneal dialysis. The mechanism of fibrosis remains unclear partly because the fibrogenic cells have not been identified with certainty. Recent studies have proposed mesothelial cells to be an important source of myofibroblasts through the epithelial-mesenchymal transition; however, confirmatory studies in vivo are lacking. Here, we show by inducible genetic fate mapping that type I collagen-producing submesothelial fibroblasts are specific progenitors of α-smooth muscle actin-positive myofibroblasts that accumulate progressively in models of peritoneal fibrosis induced by sodium hypochlorite, hyperglycemic dialysis solutions, or TGF-β1. Similar genetic mapping of Wilms' tumor-1-positive mesothelial cells indicated that peritoneal membrane disruption is repaired and replaced by surviving mesothelial cells in peritoneal injury, and not by submesothelial fibroblasts. Although primary cultures of mesothelial cells or submesothelial fibroblasts each expressed α-smooth muscle actin under the influence of TGF-β1, only submesothelial fibroblasts expressed α-smooth muscle actin after induction of peritoneal fibrosis in mice. Furthermore, pharmacologic inhibition of the PDGF receptor, which is expressed by submesothelial fibroblasts but not mesothelial cells, attenuated the peritoneal fibrosis but not the remesothelialization induced by hypochlorite. Thus, our data identify distinctive fates for injured mesothelial cells and submesothelial fibroblasts during peritoneal injury and fibrosis.
Copyright © 2014 by the American Society of Nephrology.
Figures




References
-
- Grassmann A, Gioberge S, Moeller S, Brown G: ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 20: 2587–2593, 2005 - PubMed
-
- Wu MS, Wu IW, Shih CP, Hsu KH: Establishing a platform for battling end-stage renal disease and continuing quality improvement in dialysis therapy in Taiwan - Taiwan Renal Registry Data System (TWRDS). Acta Nephrol 25: 148–153, 2011
-
- Kramer A, Stel VS, Abad Diez JM, Alonso de la Torre R, Bouzas Caamaño E, Čala S, Cao Baduell H, Castro de la Nuez P, Cernevskis H, Collart F, Couchoud C, de Meester J, Djukanovic L, Ferrer-Alamar M, Finne P, Fogarty D, de los Ángeles García Bazaga M, Garneata L, Golan E, Gonzalez Fernández R, Heaf JG, Hoitsma A, Ioannidis GA, Kolesnyk M, Kramar R, Leivestad T, Limido A, Lopot F, Macario F, Magaz Å, Martín-Escobar E, Metcalfe W, Noordzij M, Ots-Rosenberg M, Palsson R, Piñera C, Postorino M, Prutz KG, Ratkovic M, Resic H, Rodríguez Hernández A, Rutkowski B, Serdengeçti K, Yebenes TS, Spustová V, Stojceva-Taneva O, Tomilina NA, van de Luijtgaarden MWM, van Stralen KJ, Wanner C, Jager KJ: Renal replacement therapy in Europe–a summary of the 2010 ERA–EDTA Registry Annual Report. Clin Kidney J 6: 105–115, 2013 - PMC - PubMed
-
- Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517–525, 1999 - PubMed
-
- Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT, Peritoneal Biopsy Study Group : Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

