In vitro toxic effects of puff adder (Bitis arietans) venom, and their neutralization by antivenom
- PMID: 24854547
- PMCID: PMC4052254
- DOI: 10.3390/toxins6051586
In vitro toxic effects of puff adder (Bitis arietans) venom, and their neutralization by antivenom
Abstract
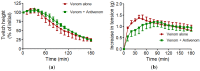
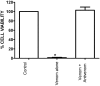
This study investigated the in vitro toxic effects of Bitis arietans venom and the ability of antivenom produced by the South African Institute of Medical Research (SAIMR) to neutralize these effects. The venom (50 µg/mL) reduced nerve-mediated twitches of the chick biventer muscle to 19% ± 2% of initial magnitude (n = 4) within 2 h. This inhibitory effect of the venom was significantly attenuated by prior incubation of tissues with SAIMR antivenom (0.864 µg/µL; 67% ± 4%; P < 0.05; n = 3-5, unpaired t-test). Addition of antivenom at t50 failed to prevent further inhibition or reverse the inhibition of twitches and responses to agonists. The myotoxic action of the venom (50 µg/mL) was evidenced by a decrease in direct twitches (30% ± 6% of the initial twitch magnitude) and increase in baseline tension (by 0.7 ± 0.3 g within 3 h) of the chick biventer. Antivenom failed to block these effects. Antivenom however prevented the venom induced cytotoxic effects on L6 skeletal muscle cells. Venom induced a marginal but significant reduction in plasma clotting times at concentrations above 7.8 µg/100 µL of plasma, indicating poor procoagulant effects. In addition, the results of western immunoblotting indicate strong immunoreactivity with venom proteins, thus warranting further detailed studies on the neutralization of the effects of individual venom toxins by antivenom.
Figures
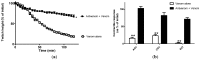


Similar articles
-
Neutralization of Bitis parviocula (Ethiopian mountain adder) venom by the South African Institute of Medical Research (SAIMR) antivenom.Rev Inst Med Trop Sao Paulo. 2011 Jul-Aug;53(4):213-7. doi: 10.1590/s0036-46652011000400007. Rev Inst Med Trop Sao Paulo. 2011. PMID: 21915465 Free PMC article.
-
Inhibition of the myotoxic activities of three African Bitis venoms (B. rhinoceros, B. arietans and B. nasicornis) by a polyvalent antivenom.Toxicon. 2010 Feb-Mar;55(2-3):536-40. doi: 10.1016/j.toxicon.2009.10.006. Epub 2009 Oct 24. Toxicon. 2010. PMID: 19857507
-
Intraspecific venom variation in the medically important puff adder (Bitis arietans): Comparative venom gland transcriptomics, in vitro venom activity and immunological recognition by antivenom.PLoS Negl Trop Dis. 2024 Oct 18;18(10):e0012570. doi: 10.1371/journal.pntd.0012570. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39423239 Free PMC article.
-
The diagnosis and management of snakebite in dogs--a southern African perspective.J S Afr Vet Assoc. 2004 Mar;75(1):7-13. doi: 10.4102/jsava.v75i1.441. J S Afr Vet Assoc. 2004. PMID: 15214688 Review.
-
Mechanism of Vipera berus venom activity and the principles of antivenom administration in treatment.Przegl Epidemiol. 2013;67(4):641-6, 729-33. Przegl Epidemiol. 2013. PMID: 24741911 Review. English, Polish.
Cited by
-
Widespread and Differential Neurotoxicity in Venoms from the Bitis Genus of Viperid Snakes.Neurotox Res. 2021 Jun;39(3):697-704. doi: 10.1007/s12640-021-00330-4. Epub 2021 Jan 11. Neurotox Res. 2021. PMID: 33428181
-
The Influence of Silver Nanoparticles Against Toxic Effects of Philodryas olfersii Venom.Int J Nanomedicine. 2021 May 25;16:3555-3564. doi: 10.2147/IJN.S293366. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34079248 Free PMC article.
-
In Vitro neurotoxicity and myotoxicity of Malaysian Naja sumatrana and Naja kaouthia venoms: Neutralization by monovalent and Neuro Polyvalent Antivenoms from Thailand.PLoS One. 2022 Sep 12;17(9):e0274488. doi: 10.1371/journal.pone.0274488. eCollection 2022. PLoS One. 2022. PMID: 36094937 Free PMC article.
-
The effect of physiological levels of South African puff adder (Bitis arietans) snake venom on blood cells: an in vitro model.Sci Rep. 2016 Oct 24;6:35988. doi: 10.1038/srep35988. Sci Rep. 2016. PMID: 27775063 Free PMC article.
-
Antiprotozoal Effect of Snake Venoms and Their Fractions: A Systematic Review.Pathogens. 2021 Dec 16;10(12):1632. doi: 10.3390/pathogens10121632. Pathogens. 2021. PMID: 34959587 Free PMC article. Review.
References
-
- Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
-
- Vulfius C.A., Gorbacheva E.V., Starkov V.G., Osipov A.V., Kasheverov I.E., Andreeva T.V., Astashev M.E., Tsetlin V.I., Utkin Y.N. An unusual phospholipase A(2) from puff adder Bitis arietans venom—A novel blocker of nicotinic acetylcholine receptors. Toxicon. 2011;57:787–793. doi: 10.1016/j.toxicon.2011.02.013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

