[Joint serum tumor markers serve as survival predictive model of erlotinib in the treatment of recurrent non-small cell lung cancer]
- PMID: 24854556
- PMCID: PMC6000447
- DOI: 10.3779/j.issn.1009-3419.2014.05.05
[Joint serum tumor markers serve as survival predictive model of erlotinib in the treatment of recurrent non-small cell lung cancer]
Abstract
Background and objective: Molecular targeting therapy is the direction of individualized treatment of lung cancer, scholars has been established targeted therapy prediction models which provide more guidance for clinical individual therapy. This study investigated the relationship among pulmonary surfactant-associated protein D (SP-D), transforming growth factor α (TGF-α), matrix metalloproteinase 9 (MMP-9), tissue polypeptide specific antigen (TPS), and Krebs von den Lungen-6 (KL-6) and response as well as survival in the patients with recurrent non-small cell lung cancer, which Erlotinib was as second line treatment after failure to chemotherapy. This study also established a predictive prognostic model.
Methods: Serum levels of SP-D, TGF-α, MMP-9, TPS, and KL-6 in 114 patients before erlotinib treatment were detected by ELISA method. Combined with clinical factors, these levels were used to investigate the relationship with efficacy in erlotinib treatment and construct a predicted prognostic model by Kaplan-Meier curve and Cox proportional hazard model multivariate analysis.
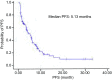
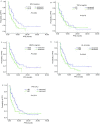
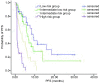
Results: The objective response rate (ORR) and disease control rate (DCR) in the 114 patients, were 22.8% (26/114) and 72.8% (83/114), to Erlotinib treatment respectively. The median progression-free survival (PFS) and one year survival rate with Erlotinib treatment were 5.13 months and 69.3%, respectively. Patients in the SP-D>110 ng/mL group exhibited more ORR (33.3% vs 13.3%, P=0.011) and DCR (83.3% vs 63.3%, P=0.017) than those in the ≤110 ng/mL group. Patients in the MMP-9≤535 ng/mL group showed more DCR (83.9%) than those in the >535 ng/mL group (62.1%) (P=0.009). Patients in the TPS<80 U/L group showed more DCR (82.4%) than those in the ≥80 U/L group (55.0%) (P=0.002). The SP-D>110 ng/mL (5.95 months vs 3.25 months, P=0.009), MMP-9≤535 ng/mL (5.83 months vs 3.47 months, P=0.046), KL-6<500 U/mL (6.03 months vs 3.40 months, P=0.040), and TPS<80 U/L (6.15 months vs 2.42 months, P=0.014) groups showed better PFS. Multivariate analysis showed that current or ever-smoker, wild style of EGFR status, progression after prior chemotherapy, absence of skin rash, elevated serum LDH level, and TPS≥80 U/L were independent adverse prognostic factors for PFS. These six factors were used in the prognostic model. Patients were categorized into four prognosis risk groups based on the prognostic index from the model, namely, low risk, intermediate low risk, intermediate risk, and high risk groups. The median PFS of good, intermediate, poor, and very poor prognosis groups were 9.12, 6.88, 3.52, and 0.93 months (P<0.001), respectively.
Conclusions: The prognostic model based on clinical parameters with TPS will be useful in identifying patients who might be most likely to benefit from Erlotinib therapy in the patients with recurrent non-small cell lung cancer.
背景与目的 分子靶向治疗是肺癌个体化治疗的方向,目前已有学者建立靶向治疗预测模型,为临床个体化治疗提供更多的指导。本研究探讨血清肺表面活性物质相关蛋白(pulmonary surfactant-associated protein D, SP-D)、转化生长因子-α(transforming growthfactor α, TGF-α)、基质金属蛋白-9(matrix metalloproteinase 9, MMP-9)、组织多肽特异性抗原(tissue polypeptide specific antigen, TPS)、肺腺癌相关抗原(Krebs von den Lungen-6, KL-6)与晚期复治非小细胞肺癌(non-small cell lung cancer, NSCLC)治疗疗效及生存的关系,并构建生存预测模型。方法 采用酶联免疫吸附法(enzyme-linked immuno sorbent assay, ELISA)检测114例晚期复治NSCLC患者治疗前外周血清中SP-D、TGF-α、MMP-9、TPS、KL-6含量,结合临床因素分析与厄洛替尼治疗疗效的关系,采用Kaplan-Meier生存曲线、Cox多因素生存分析模型进行单因素和多因素分析,并构建生存预测模型。结果 114例患者厄洛替尼治疗总有效率为22.8%,稳定率为72.8%,中位无疾病进展时间(progression-free survival, PFS)为5.13个月,1年生存率为69.3%。SP-D>110 ng/mL组的有效率及稳定率均高于≤110 ng/mL组(P=0.011, P=0.017),MMP-9≤535 ng/mL的稳定率高于>535 ng/mL组(P=0.009)。TPS <80 U/L组的稳定率要高于≥80 U/L组(P=0.002)。SP-D>110 ng/mL组的mPFS长于≤110 ng/mL组(5.95个月 vs 3.25个月,P=0.009),MMP-9≤535 ng/mL的mPFS要长于>535 ng/mL组(5.83个月 vs 3.47个月,P=0.046),KL-6中<500 U/mL组要优于≥500 U/mL组(6.03个月 vs 3.40个月,P=0.040),TPS<80 U/L组的mPFS要长于≥80 U/L组(6.15个月 vs 2.42个月,P=0.014)。多因素分析显示吸烟史、EGFR基因野生型、末次化疗疗效进展、厄洛替尼治疗期间无皮疹、LDH升高和TPS≥80 U/L是PFS不佳的独立影响因素。通过建立预后预测模型,根据患者的预后指数可分成4组:低危组、中低危组、中危组和高危组,中位PFS分别是9.12个月、6.88个月、3.52个月和0.93个月,组间差异具有统计学意义(P<0.001)。结论 血清肿瘤标志物TPS水平联合患者临床特征建立预测厄洛替尼治疗晚期复治NSCLC生存模型,在临床上有一定的指导意义。
Figures
References
-
- Hao J, Chen WQ. 2012 Chinese cancer registry annual repor. Beijing: Military Medical Science Publication; 2012. pp. 1–301.
- 郝 捷, 陈 万青. 2012中国肿瘤登记年报. 北京: 军事医学科学出版社; 2012. pp. 1–301.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

