Tuning of the copper-thioether bond in tetradentate N₃S(thioether) ligands; O-O bond reductive cleavage via a [Cu(II)₂(μ-1,2-peroxo)]²⁺/[Cu(III)₂(μ-oxo)₂]²⁺ equilibrium
- PMID: 24854766
- PMCID: PMC4063178
- DOI: 10.1021/ja502974c
Tuning of the copper-thioether bond in tetradentate N₃S(thioether) ligands; O-O bond reductive cleavage via a [Cu(II)₂(μ-1,2-peroxo)]²⁺/[Cu(III)₂(μ-oxo)₂]²⁺ equilibrium
Abstract
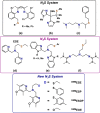
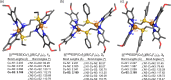
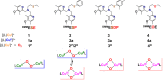
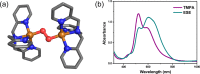
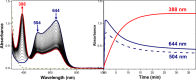
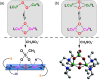
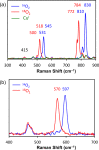
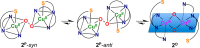
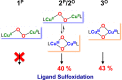
Current interest in copper/dioxygen reactivity includes the influence of thioether sulfur ligation, as it concerns the formation, structures, and properties of derived copper-dioxygen complexes. Here, we report on the chemistry of {L-Cu(I)}2-(O2) species L = (DMM)ESE, (DMM)ESP, and (DMM)ESDP, which are N3S(thioether)-based ligands varied in the nature of a substituent on the S atom, along with a related N3O(ether) (EOE) ligand. Cu(I) and Cu(II) complexes have been synthesized and crystallographically characterized. Copper(I) complexes are dimeric in the solid state, [{L-Cu(I)}2](B(C6F5)4)2, however are shown by diffusion-ordered NMR spectroscopy to be mononuclear in solution. Copper(II) complexes with a general formulation [L-Cu(II)(X)](n+) {X = ClO4(-), n = 1, or X = H2O, n = 2} exhibit distorted square pyramidal coordination geometries and progressively weaker axial thioether ligation across the series. Oxygenation (-130 °C) of {((DMM)ESE)Cu(I)}(+) results in the formation of a trans-μ-1,2-peroxodicopper(II) species [{((DMM)ESE)Cu(II)}2(μ-1,2-O2(2-))](2+) (1(P)). Weakening the Cu-S bond via a change to the thioether donor found in (DMM)ESP leads to the initial formation of [{((DMM)ESP)Cu(II)}2(μ-1,2-O2(2-))](2+) (2(P)) that subsequently isomerizes to a bis-μ-oxodicopper(III) complex, [{((DMM)ESP)Cu(III)}2(μ-O(2-))2](2+) (2(O)), with 2(P) and 2(O) in equilibrium (K(eq) = [2(O)]/[2(P)] = 2.6 at -130 °C). Formulations for these Cu/O2 adducts were confirmed by resonance Raman (rR) spectroscopy. This solution mixture is sensitive to the addition of methylsulfonate, which shifts the equilibrium toward the bis-μ-oxo isomer. Further weakening of the Cu-S bond in (DMM)ESDP or substitution with an ether donor in (DMM)EOE leads to only a bis-μ-oxo species (3(O) and 4(O), respectively). Reactivity studies indicate that the bis-μ-oxodicopper(III) species (2(O), 3(O)) and not the trans-peroxo isomers (1(P) and 2(P)) are responsible for the observed ligand sulfoxidation. Our findings concerning the existence of the 2(P)/2(O) equilibrium contrast with previously established ligand-Cu(I)/O2 reactivity and possible implications are discussed.
Figures
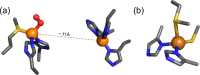

References
-
- Solomon E. I.; Heppner D. E.; Johnston E. M.; Ginsbach J. W.; Cirera J.; Qayyum M.; Kieber-Emmons M. T.; Kjaergaard C. H.; Hadt R. G.; Tian L. Chem. Rev. 2014, 114, 3659–3853. - PMC - PubMed
- Peterson R. L.; Kim S.; Karlin K. D. In Comprehensive Inorganic Chemistry II; 2nd ed.; Elsevier: Amsterdam, 2013; pp 149–177.
-
- Solomon E. I.; Szilagyi R. K.; George S. D.; Basumallick L. Chem. Rev. 2004, 104, 419–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

