An Improved Antagonist Radiotracer for the κ-Opioid Receptor: Synthesis and Characterization of (11)C-LY2459989
- PMID: 24854795
- PMCID: PMC4826283
- DOI: 10.2967/jnumed.114.138701
An Improved Antagonist Radiotracer for the κ-Opioid Receptor: Synthesis and Characterization of (11)C-LY2459989
Abstract
The κ-opioid receptors (KORs) are implicated in several neuropsychiatric diseases and addictive disorders. PET with radioligands provides a means to image the KOR in vivo and investigate its function in health and disease. The purpose of this study was to develop the selective KOR antagonist (11)C-LY2459989 as a PET radioligand and characterize its imaging performance in nonhuman primates.
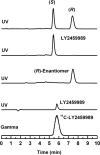
Methods: LY2459989 was synthesized and assayed for in vitro binding to opioid receptors. Ex vivo studies in rodents were conducted to assess its potential as a tracer candidate. (11)C-LY2459989 was synthesized by reaction of its iodophenyl precursor with (11)C-cyanide, followed by partial hydrolysis of the resulting (11)C-cyanophenyl intermediate. Imaging experiments with (11)C-LY2459989 were performed in rhesus monkeys with arterial input function measurement. Imaging data were analyzed with kinetic models to derive in vivo binding parameters.
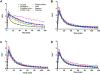
Results: LY2459989 is a full antagonist with high binding affinity and selectivity for KOR (0.18, 7.68, and 91.3 nM, respectively, for κ, μ, and δ receptors). Ex vivo studies in rats indicated LY2459989 as an appropriate tracer candidate with high specific binding signals and confirmed its KOR binding selectivity in vivo. (11)C-LY2459989 was synthesized in high radiochemical purity and good specific activity. In rhesus monkeys, (11)C-LY2459989 displayed a fast rate of peripheral metabolism. Similarly, (11)C-LY2459989 displayed fast uptake kinetics in the brain and an uptake pattern consistent with the distribution of KOR in primates. Pretreatment with naloxone (1 mg/kg, intravenously) resulted in a uniform distribution of radioactivity in the brain. Further, specific binding of (11)C-LY2459989 was dose-dependently reduced by the selective KOR antagonist LY2456302 and the unlabeled LY2459989. Regional binding potential values derived from the multilinear analysis-1 (MA1) method, as a measure of in vivo specific binding signal, were 2.18, 1.39, 1.08, 1.04, 1.03, 0.59, 0.51, and 0.50, respectively, for the globus pallidus, cingulate cortex, insula, caudate, putamen, frontal cortex, temporal cortex, and thalamus.
Conclusion: The novel PET radioligand (11)C-LY2459989 displayed favorable pharmacokinetic properties, a specific and KOR-selective binding profile, and high specific binding signals in vivo, thus making it a promising PET imaging agent for KOR.
Keywords: PET; antagonist; kappa opioid receptor; radioligand; synthesis and evaluation.
© 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Figures

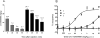



Similar articles
-
Novel 18F-Labeled κ-Opioid Receptor Antagonist as PET Radiotracer: Synthesis and In Vivo Evaluation of 18F-LY2459989 in Nonhuman Primates.J Nucl Med. 2018 Jan;59(1):140-146. doi: 10.2967/jnumed.117.195586. Epub 2017 Jul 26. J Nucl Med. 2018. PMID: 28747521 Free PMC article.
-
Synthesis and evaluation of 11C-LY2795050 as a κ-opioid receptor antagonist radiotracer for PET imaging.J Nucl Med. 2013 Mar;54(3):455-63. doi: 10.2967/jnumed.112.109512. Epub 2013 Jan 25. J Nucl Med. 2013. PMID: 23353688 Free PMC article.
-
Novel Kappa Opioid Receptor Agonist as Improved PET Radiotracer: Development and in Vivo Evaluation.Mol Pharm. 2019 Apr 1;16(4):1523-1531. doi: 10.1021/acs.molpharmaceut.8b01209. Epub 2019 Feb 28. Mol Pharm. 2019. PMID: 30726092
-
Imaging Kappa Opioid Receptors in the Living Brain with Positron Emission Tomography.Handb Exp Pharmacol. 2022;271:547-577. doi: 10.1007/164_2021_498. Handb Exp Pharmacol. 2022. PMID: 34363128 Review.
-
[Carbonyl-11C](S)-3-chloro-4-(4-((2-(pyridine-3-yl)pyrrolidin-1-yl)methyl)phenoxy)benzamide.2013 Mar 21 [updated 2013 May 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2013 Mar 21 [updated 2013 May 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23700645 Free Books & Documents. Review.
Cited by
-
A Survey of Molecular Imaging of Opioid Receptors.Molecules. 2019 Nov 19;24(22):4190. doi: 10.3390/molecules24224190. Molecules. 2019. PMID: 31752279 Free PMC article. Review.
-
Relevance of the Kappa Dynorphin System to Schizophrenia and Its Therapeutics.J Psychiatr Brain Sci. 2021;6:e210015. doi: 10.20900/jpbs.20210015. Epub 2021 Aug 24. J Psychiatr Brain Sci. 2021. PMID: 34532594 Free PMC article.
-
PET imaging of kappa opioid receptors and receptor expression quantified in neuron-derived extracellular vesicles in socially housed female and male cynomolgus macaques.Neuropsychopharmacology. 2023 Jan;48(2):410-417. doi: 10.1038/s41386-022-01444-9. Epub 2022 Sep 13. Neuropsychopharmacology. 2023. PMID: 36100655 Free PMC article.
-
Strategies for the Production of [11C]LY2795050 for Clinical Use.Org Process Res Dev. 2023 Feb 17;27(2):373-381. doi: 10.1021/acs.oprd.2c00388. Epub 2023 Feb 8. Org Process Res Dev. 2023. PMID: 36874204 Free PMC article.
-
Targeting opioid dysregulation in depression for the development of novel therapeutics.Pharmacol Ther. 2019 Sep;201:51-76. doi: 10.1016/j.pharmthera.2019.04.009. Epub 2019 Apr 30. Pharmacol Ther. 2019. PMID: 31051197 Free PMC article. Review.
References
-
- Dhawan BN, Cesselin F, Raghubir R, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. - PubMed
-
- Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23:121–145. - PubMed
-
- Hiller JM, Fan LQ. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem Res. 1996;21:1333–1345. - PubMed
-
- Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. μ-, δ- and κ-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer’s disease patients. Brain Res. 2001;893:121–134. - PubMed
-
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
