Essential role of the chaperonin CCT in rod outer segment biogenesis
- PMID: 24854858
- PMCID: PMC4062400
- DOI: 10.1167/iovs.14-13889
Essential role of the chaperonin CCT in rod outer segment biogenesis
Abstract
Purpose: While some evidence suggests an essential role for the chaperonin containing t-complex protein 1 (CCT) in ciliogenesis, this function remains poorly understood mechanistically. We used transgenic mice, previously generated in our lab, and characterized by a genetically-induced suppression of CCT in rod photoreceptors as well as a malformation of the rod sensory cilia, the outer segments, to gain new insights into this underlying molecular mechanism.
Methods: The CCT activity in rod photoreceptors of mice was suppressed by overexpressing the chaperonin inhibitor, phosducin-like protein short, and the ensuing changes of cellular morphology were analyzed by light and electron microscopy. Protein expression levels were studied by fluorescent microscopy and Western blotting.
Results: Suppressing the chaperonin made the photoreceptors incompetent to build their outer segments. Specifically, the CCT-deficient rods appeared unable to expand the outer segment plasma membrane, and accommodate growth of this compartment. Seeking the molecular mechanisms underlying such a shortcoming, we found that the affected rods could not express normal levels of Bardet-Biedl Syndrome (BBS) proteins 2, 5, and 7 and, owing to that deficiency, were unable to assemble the BBSome, a multisubunit complex responsible for ciliary trafficking. A similar effect in response to the chaperonin suppression was also observed in cultured ciliated cells.
Conclusions: Our data provide new evidence indicating the essential role of the chaperonin CCT in the biogenesis of vertebrate photoreceptor sensory cilia, and suggest that it may be due to the direct participation of the chaperonin in the posttranslational processing of selected BBS proteins and assembly of the BBSome.
Keywords: BBSome; Bardet-Biedl Syndrome; chaperonin CCT; outer segments; rods.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures






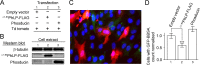
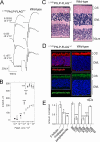
Similar articles
-
Disruption of the chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival.Mol Cell Proteomics. 2011 Jan;10(1):M110.000570. doi: 10.1074/mcp.M110.000570. Epub 2010 Sep 17. Mol Cell Proteomics. 2011. PMID: 20852191 Free PMC article.
-
BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly.Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1488-93. doi: 10.1073/pnas.0910268107. Epub 2010 Jan 4. Proc Natl Acad Sci U S A. 2010. PMID: 20080638 Free PMC article.
-
BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking.J Cell Sci. 2013 Jun 1;126(Pt 11):2372-80. doi: 10.1242/jcs.111740. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572516 Free PMC article.
-
Bardet-Biedl syndrome: The pleiotropic role of the chaperonin-like BBS6, 10, and 12 proteins.Am J Med Genet C Semin Med Genet. 2022 Mar;190(1):9-19. doi: 10.1002/ajmg.c.31970. Epub 2022 Apr 4. Am J Med Genet C Semin Med Genet. 2022. PMID: 35373910 Free PMC article. Review.
-
Bardet-Biedl Syndrome as a Chaperonopathy: Dissecting the Major Role of Chaperonin-Like BBS Proteins (BBS6-BBS10-BBS12).Front Mol Biosci. 2017 Jul 31;4:55. doi: 10.3389/fmolb.2017.00055. eCollection 2017. Front Mol Biosci. 2017. PMID: 28824921 Free PMC article. Review.
Cited by
-
Quantitative Proteomic Analyses Identify ABA-Related Proteins and Signal Pathways in Maize Leaves under Drought Conditions.Front Plant Sci. 2016 Dec 8;7:1827. doi: 10.3389/fpls.2016.01827. eCollection 2016. Front Plant Sci. 2016. PMID: 28008332 Free PMC article.
-
Usher syndrome proteins ADGRV1 (USH2C) and CIB2 (USH1J) interact and share a common interactome containing TRiC/CCT-BBS chaperonins.Front Cell Dev Biol. 2023 Jun 22;11:1199069. doi: 10.3389/fcell.2023.1199069. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37427378 Free PMC article.
-
The Molecular Architecture of Native BBSome Obtained by an Integrated Structural Approach.Structure. 2019 Sep 3;27(9):1384-1394.e4. doi: 10.1016/j.str.2019.06.006. Epub 2019 Jul 11. Structure. 2019. PMID: 31303482 Free PMC article.
-
Correlation of ER stress and retinal degeneration in tubby mice.Exp Eye Res. 2015 Nov;140:130-138. doi: 10.1016/j.exer.2015.08.022. Epub 2015 Sep 9. Exp Eye Res. 2015. PMID: 26325328 Free PMC article.
-
Compound heterozygous mutations in a mouse model of Leber congenital amaurosis reveal the role of CCT2 in photoreceptor maintenance.Commun Biol. 2024 Jun 3;7(1):676. doi: 10.1038/s42003-024-06384-2. Commun Biol. 2024. PMID: 38830954 Free PMC article.
References
-
- Munoz IG, Yebenes H, Zhou M, et al. Crystal structure of the open conformation of the mammalian chaperonin CCT in complex with tubulin. Nat Struct Mol Biol. 2011; 18: 14–19 - PubMed
-
- Camasses A, Bogdanova A, Shevchenko A, Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell. 2003; 12: 87–100 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

