Positive selection for bone morphogenetic protein receptor type-IB promotes differentiation and specification of human adipose-derived stromal cells toward an osteogenic lineage
- PMID: 24854876
- PMCID: PMC4229710
- DOI: 10.1089/ten.TEA.2014.0101
Positive selection for bone morphogenetic protein receptor type-IB promotes differentiation and specification of human adipose-derived stromal cells toward an osteogenic lineage
Abstract
Background: Adipose tissue represents an abundant and easily accessible source of multipotent cells that may serve as an excellent building block for tissue engineering. However, adipose-derived stromal cells (ASCs) are a heterogeneous group and subpopulations may be identified with enhanced osteogenic potential.
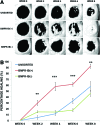
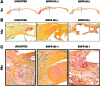
Methods: Human ASC subpopulations were prospectively isolated based on expression of bone morphogenetic protein receptor type-IB (BMPR-IB). Unsorted, BMPR-IB(+), and BMPR-IB(-) cells were analyzed for their osteogenic capacity through histological staining and gene expression. To evaluate their in vivo osteogenic potential, critical-sized calvarial defects were created in immunocompromised mice and treated with unsorted, BMPR-IB(+), or BMPR-IB(-) cells. Healing was assessed using microcomputed tomography and pentachrome staining of specimens at 8 weeks.
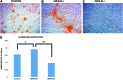
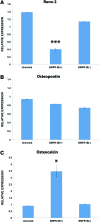
Results: Increased osteogenic differentiation was noted in the BMPR-IB(+) subpopulation, as demonstrated by alkaline phosphatase staining at day 7 and extracellular matrix mineralization with Alizarin red staining at day 14. This was also associated with increased expression for osteocalcin, a late marker of osteogenesis. Radiographic analysis demonstrated significantly enhanced healing of critical-sized calvarial defects treated with BMPR-IB(+) ASCs compared with unsorted or BMPR-IB(-) cells. This was confirmed through pentachrome staining, which revealed more robust bone regeneration in the BMPR-IB(+) group.
Conclusion: BMPR-IB(+) human ASCs have an enhanced ability to form bone both in vitro and in vivo. These data suggest that positive selection for BMPR-IB(+) and manipulation of the BMP pathway in these cells may yield a highly osteogenic subpopulation of cells for bone tissue engineering.
Figures


References
-
- Chapekar M.S.Tissue engineering: challenges and opportunities. J Biomed Mater Res 53,617, 2000 - PubMed
-
- McArdle A., Lo D.D., Hyun J.S., Senarath-Yapa K., Chung M.T., Wan D.C., et al. Discussion: a report of the ASPS task force on regenerative medicine: opportunities for plastic surgery. Plast Reconstr Surg 131,400, 2013 - PubMed
-
- Gimble J.M., and Guilak F.Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 58,137, 2003 - PubMed
-
- Cowan C.M., Shi Y.Y., Aalami O.O., Chou Y.F., Mari C., Thomas R., et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22,560, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

