Understanding pharmaceutical quality by design
- PMID: 24854893
- PMCID: PMC4070262
- DOI: 10.1208/s12248-014-9598-3
Understanding pharmaceutical quality by design
Abstract
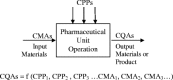
This review further clarifies the concept of pharmaceutical quality by design (QbD) and describes its objectives. QbD elements include the following: (1) a quality target product profile (QTPP) that identifies the critical quality attributes (CQAs) of the drug product; (2) product design and understanding including identification of critical material attributes (CMAs); (3) process design and understanding including identification of critical process parameters (CPPs), linking CMAs and CPPs to CQAs; (4) a control strategy that includes specifications for the drug substance(s), excipient(s), and drug product as well as controls for each step of the manufacturing process; and (5) process capability and continual improvement. QbD tools and studies include prior knowledge, risk assessment, mechanistic models, design of experiments (DoE) and data analysis, and process analytical technology (PAT). As the pharmaceutical industry moves toward the implementation of pharmaceutical QbD, a common terminology, understanding of concepts and expectations are necessary. This understanding will facilitate better communication between those involved in risk-based drug development and drug application review.
Figures
References
-
- Juran JM. Juran on quality by design: the new steps for planning quality into goods and services. New York: The Free Press; 1992.
-
- Woodcock J. The concept of pharmaceutical quality. Am Pharm Rev 2004; 1–3.
-
- U. S. Food and Drug Administration. Guidance for Industry: Q8 (2) Pharmaceutical Development. 2009
-
- U. S. Food and Drug Administration. Guidance for Industry: Q9 Quality Risk Management. 2006.
-
- U. S. Food and Drug Administration. Guidance for Industry: Q10 pharmaceutical quality system. 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials