Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma
- PMID: 24855336
- PMCID: PMC4019628
- DOI: 10.2147/OPTH.S59932
Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma
Abstract
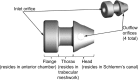
Purpose: The purpose of this study was to compare outcomes of subjects with open-angle glaucoma (OAG) not controlled on one medication who underwent either implantation of two iStent inject (®) trabecular micro-bypass devices or received medical therapy consisting of a fixed combination of latanoprost/timolol.
Patients and methods: Of 192 subjects who qualified for the study and were enrolled, 94 were randomized to surgery with implantation of two iStent inject(®) devices in the treated eye and 98 to receive medical therapy.
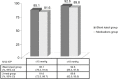
Results: At the month 12 visit, 94.7% of eyes (89/94) in the stent group reported an unmedicated intraocular pressure (IOP) reduction of ≥20% versus baseline unmedicated IOP, and 91.8% of eyes (88/98) in the medical therapy group reported an IOP reduction ≥20% versus baseline unmedicated IOP. A 17.5% between-group treatment difference in favor of the iStent inject group was statistically significant (P=0.02) at the ≥50% level of IOP reduction. An IOP ≤18 mmHg was reported in 92.6% of eyes (87/94) in the iStent inject group and 89.8% of eyes (88/98) in the medical therapy group. Mean (standard deviation) IOP decreases from screening of 8.1 (2.6) mmHg and 7.3 (2.2) mmHg were reported in the iStent inject and medical therapy groups, respectively. A high safety profile was also noted in this study in both the iStent inject and medical therapy groups, as measured by stable best corrected visual acuity, cup-to-disc ratio, and adverse events.
Conclusion: These data show that the use of iStent inject is at least as effective as two medications, with the clinical benefit of reducing medication burden and assuring continuous treatment with full compliance to implant therapy as well as having a highly favorable safety profile.
Keywords: IOP reduction; OAG; ab interno; intraocular pressure; trabecular bypass.
Figures

References
-
- Liesegang TJ. Conjunctival changes associated with glaucoma therapy: implications for the external disease consultant and the treatment of glaucoma. Cornea. 1998;17(6):574–583. - PubMed
-
- Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, Lamielle H. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25(5):453–464. - PubMed
-
- Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE, US iStent Study Group Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. - PubMed
-
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: 2-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. - PubMed
-
- Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–412. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

