Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells
- PMID: 24855338
- PMCID: PMC4020885
- DOI: 10.2147/DDDT.S58414
Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells
Abstract
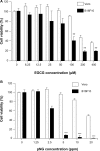
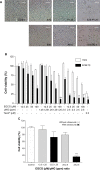
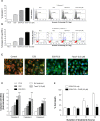
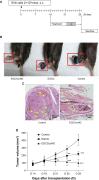
(-)-Epigallocatechin-3-gallate (EGCG), the major bioactive constituent in green tea, has been reported to effectively inhibit the formation and development of tumors. To maximize the effectiveness of EGCG, we attached it to nanogold particles (EGCG-pNG) in various ratios to examine in vitro cytotoxicity and in vivo anti-cancer activity. EGCG-pNG showed improved anti-cancer efficacy in B16F10 murine melanoma cells; the cytotoxic effect in the melanoma cells treated with EGCG-pNG was 4.91 times higher than those treated with EGCG. The enhancement is achieved through mitochondrial pathway-mediated apoptosis as determined by annexin V assay, JC-10 staining, and caspase-3, -8, -9 activity assay. Moreover, EGCG-pNG was 1.66 times more potent than EGCG for inhibition of tumor growth in a murine melanoma model. In the hemolysis assay, the pNG surface conjugated with EGCG is most likely the key factor that contributes to the decreased release of hemoglobin from human red blood cells.
Keywords: EGCG; anticancer; gold nanoparticles; melanoma.
Figures
References
-
- Markovic SN, Erickson LA, Rao RD, et al. Melanoma Study Group of the Mayo Clinic Cancer Center Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82(3):364–380. - PubMed
-
- Pusceddu S, Bajetta E, Carcangiu ML, Formisano B, Ducceschi M, Buzzoni R. A literature overview of primary cervical malignant melanoma: an exceedingly rare cancer. Crit Rev Oncol Hematol. 2012;81(2):185–195. - PubMed
-
- Vikey AK, Vikey D. Primary malignant melanoma, of head and neck: a comprehensive review of literature. Oral Oncol. 2012;48(5):399–403. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

