Novel zinc-binding site in the E2 domain regulates amyloid precursor-like protein 1 (APLP1) oligomerization
- PMID: 24855651
- PMCID: PMC4081940
- DOI: 10.1074/jbc.M114.570382
Novel zinc-binding site in the E2 domain regulates amyloid precursor-like protein 1 (APLP1) oligomerization
Abstract
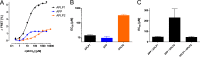
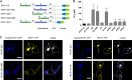
The amyloid precursor protein (APP) and the APP-like proteins 1 and 2 (APLP1 and APLP2) are a family of multidomain transmembrane proteins possessing homo- and heterotypic contact sites in their ectodomains. We previously reported that divalent metal ions dictate the conformation of the extracellular APP E2 domain (Dahms, S. O., Könnig, I., Roeser, D., Gührs, K.-H., Mayer, M. C., Kaden, D., Multhaup, G., and Than, M. E. (2012) J. Mol. Biol. 416, 438-452), but unresolved is the nature and functional importance of metal ion binding to APLP1 and APLP2. We found here that zinc ions bound to APP and APLP1 E2 domains and mediated their oligomerization, whereas the APLP2 E2 domain interacted more weakly with zinc possessing a less surface-exposed zinc-binding site, and stayed monomeric. Copper ions bound to E2 domains of all three proteins. Fluorescence resonance energy transfer (FRET) analyses examined the effect of metal ion binding to APP and APLPs in the cellular context in real time. Zinc ions specifically induced APP and APLP1 oligomerization and forced APLP1 into multimeric clusters at the plasma membrane consistent with zinc concentrations in the blood and brain. The observed effects were mediated by a novel zinc-binding site within the APLP1 E2 domain as APLP1 deletion mutants revealed. Based upon its cellular localization and its dominant response to zinc ions, APLP1 is mainly affected by extracellular zinc among the APP family proteins. We conclude that zinc binding and APP/APLP oligomerization are intimately linked, and we propose that this represents a novel mechanism for regulating APP/APLP protein function at the molecular level.
Keywords: Amyloid Precursor Protein (APP); Fluorescence Resonance Energy Transfer (FRET); Mass Spectrometry (MS); Metalloprotein; Zinc.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Coulson E. J., Paliga K., Beyreuther K., Masters C. L. (2000) What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem. Int. 36, 175–184 - PubMed
-
- Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 - PubMed
-
- Eggert S., Paliga K., Soba P., Evin G., Masters C. L., Weidemann A., Beyreuther K. (2004) The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ϵ-like cleavages: modulation of APLP-1 processing by N-glycosylation. J. Biol. Chem. 279, 18146–18156 - PubMed
-
- Li Q., Südhof T. C. (2004) Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J. Biol. Chem. 279, 10542–10550 - PubMed
-
- Kuhn P.-H., Koroniak K., Hogl S., Colombo A., Zeitschel U., Willem M., Volbracht C., Schepers U., Imhof A., Hoffmeister A., Haass C., Rossner S., Bräse S., Lichtenthaler S. F. (2012) Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 31, 3157–3168 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

