Functions of caspase 8: the identified and the mysterious
- PMID: 24856110
- PMCID: PMC4099255
- DOI: 10.1016/j.smim.2014.03.005
Functions of caspase 8: the identified and the mysterious
Abstract
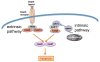
Initially discovered as an initiator protease in apoptosis mediated by death receptors, caspase-8 is now known to have an apparently confounding opposing effect in securing cell survival. It is required to allow mouse embryo survival, and the survival of hematopoietic cells during their development and activation. Classic models in which caspase-8 is depleted or inhibited frequently result in inhibition of apoptosis, and conversion to death through a necrotic pathway. This bewildering switch is now known to be driven by activation of a pathway dependent on protein kinases of the RIP family, which engage a pathway known as necroptosis. If caspase-8 does not control this pathway, necrotic death results. The pro-apoptotic and pro-survival functions of caspase-8 are regulated by a specific interaction with the pseudo-caspase cFLIP, and it is thought that the heterocomplex between these two partners alters the substrate specificity of caspase-8 in favor of inactivating components of the RIP kinase pathway. The description of how caspase-8 and cFLIP coordinate the switch between apoptosis and survival is just beginning. The mechanism is not known, the differential targets are not known, and the reason of why an apoptotic initiator has been co-opted as a critical survival factor is only guessed at. Elucidating these unknowns will be important in understanding mechanisms and possible therapeutic targets in autoimmune, inflammatory, and metastatic diseases.
Keywords: Apoptosis; Autophagy; Necroptosis; Necrosis.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
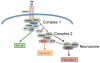
References
-
- Horvitz HR. Nobel lecture. Worms, life and death. Biosci Rep. 2003;23(5–6):239–303. - PubMed
-
- Alnemri E, et al. Human ICE/CED-3 protease nomenclature [letter] Cell. 1996;87:171. - PubMed
-
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–911. - PubMed
-
- Thornberry N, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272(29):17907–11. - PubMed
-
- Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272(41):25719–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

