Intrinsic conformational plasticity of native EmrE provides a pathway for multidrug resistance
- PMID: 24856154
- PMCID: PMC4063181
- DOI: 10.1021/ja503145x
Intrinsic conformational plasticity of native EmrE provides a pathway for multidrug resistance
Abstract
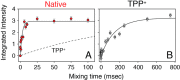
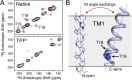
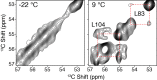
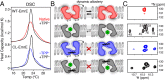
EmrE is a multidrug resistance efflux pump with specificity to a wide range of antibiotics and antiseptics. To obtain atomic-scale insight into the attributes of the native state that encodes the broad specificity, we used a hybrid of solution and solid-state NMR methods in lipid bilayers and bicelles. Our results indicate that the native EmrE dimer oscillates between inward and outward facing structural conformations at an exchange rate (k(ex)) of ~300 s(-1) at 37 °C (millisecond motions), which is ~50-fold faster relative to the tetraphenylphosphonium (TPP(+)) substrate-bound form of the protein. These observables provide quantitative evidence that the rate-limiting step in the TPP(+) transport cycle is not the outward-inward conformational change in the absence of drug. In addition, using differential scanning calorimetry, we found that the width of the gel-to-liquid crystalline phase transition was 2 °C broader in the absence of the TPP(+) substrate versus its presence, which suggested that changes in transporter dynamics can impact the phase properties of the membrane. Interestingly, experiments with cross-linked EmrE showed that the millisecond inward-open to outward-open dynamics was not the culprit of the broadening. Instead, the calorimetry and NMR data supported the conclusion that faster time scale structural dynamics (nanosecond-microsecond) were the source and therefore impart the conformationally plastic character of native EmrE capable of binding structurally diverse substrates. These findings provide a clear example how differences in membrane protein transporter structural dynamics between drug-free and bound states can have a direct impact on the physical properties of the lipid bilayer in an allosteric fashion.
Figures



Similar articles
-
A structured loop modulates coupling between the substrate-binding and dimerization domains in the multidrug resistance transporter EmrE.J Biol Chem. 2015 Jan 9;290(2):805-14. doi: 10.1074/jbc.M114.601963. Epub 2014 Nov 18. J Biol Chem. 2015. PMID: 25406320 Free PMC article.
-
New free-exchange model of EmrE transport.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10083-E10091. doi: 10.1073/pnas.1708671114. Epub 2017 Nov 7. Proc Natl Acad Sci U S A. 2017. PMID: 29114048 Free PMC article.
-
Structure, dynamics, and substrate-induced conformational changes of the multidrug transporter EmrE in liposomes.J Biol Chem. 2010 Aug 20;285(34):26710-8. doi: 10.1074/jbc.M110.132621. Epub 2010 Jun 15. J Biol Chem. 2010. PMID: 20551331 Free PMC article.
-
Comparison of three structures of the multidrug transporter EmrE.Curr Opin Struct Biol. 2006 Aug;16(4):457-64. doi: 10.1016/j.sbi.2006.06.005. Epub 2006 Jul 7. Curr Opin Struct Biol. 2006. PMID: 16828280 Review.
-
Analyzing conformational changes in the transport cycle of EmrE.Curr Opin Struct Biol. 2012 Feb;22(1):38-43. doi: 10.1016/j.sbi.2011.10.004. Epub 2011 Nov 16. Curr Opin Struct Biol. 2012. PMID: 22100111 Free PMC article. Review.
Cited by
-
Solid-state NMR and membrane proteins.J Magn Reson. 2015 Apr;253:129-37. doi: 10.1016/j.jmr.2014.11.015. Epub 2014 Dec 29. J Magn Reson. 2015. PMID: 25681966 Free PMC article.
-
Enhancing the stability and homogeneity of non-ionic polymer nanodiscs by tuning electrostatic interactions.J Colloid Interface Sci. 2023 Mar 15;634:887-896. doi: 10.1016/j.jcis.2022.12.112. Epub 2022 Dec 21. J Colloid Interface Sci. 2023. PMID: 36566634 Free PMC article.
-
Characterization of nanodisc-forming peptides for membrane protein studies.J Colloid Interface Sci. 2024 Jan;653(Pt B):1402-1414. doi: 10.1016/j.jcis.2023.09.162. Epub 2023 Sep 29. J Colloid Interface Sci. 2024. PMID: 37801850 Free PMC article.
-
Opportunities and Challenges of Backbone, Sidechain, and RDC Experiments to Study Membrane Protein Dynamics in a Detergent-Free Lipid Environment Using Solution State NMR.Front Mol Biosci. 2019 Oct 25;6:103. doi: 10.3389/fmolb.2019.00103. eCollection 2019. Front Mol Biosci. 2019. PMID: 31709261 Free PMC article. Review.
-
Electrostatic lock in the transport cycle of the multidrug resistance transporter EmrE.Proc Natl Acad Sci U S A. 2018 Aug 7;115(32):E7502-E7511. doi: 10.1073/pnas.1722399115. Epub 2018 Jul 19. Proc Natl Acad Sci U S A. 2018. PMID: 30026196 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

