Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma
- PMID: 24856585
- PMCID: PMC4096698
- DOI: 10.1016/j.ccr.2014.04.021
Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma
Abstract
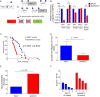
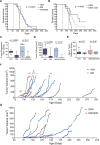
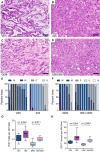
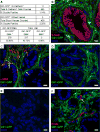
Sonic hedgehog (Shh), a soluble ligand overexpressed by neoplastic cells in pancreatic ductal adenocarcinoma (PDAC), drives formation of a fibroblast-rich desmoplastic stroma. To better understand its role in malignant progression, we deleted Shh in a well-defined mouse model of PDAC. As predicted, Shh-deficient tumors had reduced stromal content. Surprisingly, such tumors were more aggressive and exhibited undifferentiated histology, increased vascularity, and heightened proliferation--features that were fully recapitulated in control mice treated with a Smoothened inhibitor. Furthermore, administration of VEGFR blocking antibody selectively improved survival of Shh-deficient tumors, indicating that Hedgehog-driven stroma suppresses tumor growth in part by restraining tumor angiogenesis. Together, these data demonstrate that some components of the tumor stroma can act to restrain tumor growth.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Microenvironment: An exercise in restraint.Nat Rev Cancer. 2014 Jul;14(7):449. doi: 10.1038/nrc3769. Epub 2014 Jun 5. Nat Rev Cancer. 2014. PMID: 24898060 No abstract available.
-
Pancreatic cancer: surprising role for fibrosis.Nat Rev Gastroenterol Hepatol. 2014 Jul;11(7):396. doi: 10.1038/nrgastro.2014.97. Epub 2014 Jun 10. Nat Rev Gastroenterol Hepatol. 2014. PMID: 24912392 No abstract available.
-
Pancreatic cancer stroma: friend or foe?Cancer Cell. 2014 Jun 16;25(6):711-2. doi: 10.1016/j.ccr.2014.05.026. Cancer Cell. 2014. PMID: 24937454 Free PMC article.
-
[Pancreatic cancer stroma: oncologist's ally or foe?].Z Gastroenterol. 2015 Apr;53(4):337-8. doi: 10.1055/s-0034-1398955. Epub 2015 Apr 10. Z Gastroenterol. 2015. PMID: 25860583 German. No abstract available.
References
-
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. Journal of cellular biochemistry. 2007;101:887–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA136526/CA/NCI NIH HHS/United States
- CA046952/CA/NCI NIH HHS/United States
- K08 DK088945/DK/NIDDK NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- T32CA009503/CA/NCI NIH HHS/United States
- S10 RR025482/RR/NCRR NIH HHS/United States
- DK034933/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- CA169123/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- P30 DK084567/DK/NIDDK NIH HHS/United States
- P30DK050306/DK/NIDDK NIH HHS/United States
- R01 CA177857/CA/NCI NIH HHS/United States
- S10 RR025686/RR/NCRR NIH HHS/United States
- DK083111/DK/NIDDK NIH HHS/United States
- P30CA013696/CA/NCI NIH HHS/United States
- T32 CA009503/CA/NCI NIH HHS/United States
- CA177857/CA/NCI NIH HHS/United States
- R01 CA157980/CA/NCI NIH HHS/United States
- R01 CA169123/CA/NCI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- CA136526/CA/NCI NIH HHS/United States
- DP2 DK083111/DK/NIDDK NIH HHS/United States
- S10RR025686/RR/NCRR NIH HHS/United States
- DK083355/DK/NIDDK NIH HHS/United States
- R01 HL112626/HL/NHLBI NIH HHS/United States
- R01 DK083355/DK/NIDDK NIH HHS/United States
- DK088945/DK/NIDDK NIH HHS/United States
- CA157980/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

