Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival
- PMID: 24856586
- PMCID: PMC4180632
- DOI: 10.1016/j.ccr.2014.04.005
Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival
Erratum in
- Cancer Cell. 2015 Dec 14;28(6):831-3
Abstract
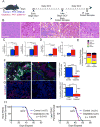
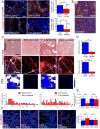
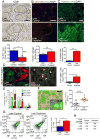
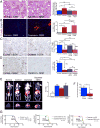
Pancreatic ductal adenocarcinoma (PDAC) is associated with marked fibrosis and stromal myofibroblasts, but their functional contribution remains unknown. Transgenic mice with the ability to delete αSMA(+) myofibroblasts in pancreatic cancer were generated. Depletion starting at either noninvasive precursor (pancreatic intraepithelial neoplasia) or the PDAC stage led to invasive, undifferentiated tumors with enhanced hypoxia, epithelial-to-mesenchymal transition, and cancer stem cells, with diminished animal survival. In PDAC patients, fewer myofibroblasts in their tumors also correlated with reduced survival. Suppressed immune surveillance with increased CD4(+)Foxp3(+) Tregs was observed in myofibroblast-depleted mouse tumors. Although myofibroblast-depleted tumors did not respond to gemcitabine, anti-CTLA4 immunotherapy reversed disease acceleration and prolonged animal survival. This study underscores the need for caution in targeting carcinoma-associated fibroblasts in PDAC.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Microenvironment: An exercise in restraint.Nat Rev Cancer. 2014 Jul;14(7):449. doi: 10.1038/nrc3769. Epub 2014 Jun 5. Nat Rev Cancer. 2014. PMID: 24898060 No abstract available.
-
Pancreatic cancer: surprising role for fibrosis.Nat Rev Gastroenterol Hepatol. 2014 Jul;11(7):396. doi: 10.1038/nrgastro.2014.97. Epub 2014 Jun 10. Nat Rev Gastroenterol Hepatol. 2014. PMID: 24912392 No abstract available.
-
[Pancreatic cancer stroma: oncologist's ally or foe?].Z Gastroenterol. 2015 Apr;53(4):337-8. doi: 10.1055/s-0034-1398955. Epub 2015 Apr 10. Z Gastroenterol. 2015. PMID: 25860583 German. No abstract available.
References
-
- Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nature medicine. 2013;19:1410–1422. - PubMed
-
- Angeli F, Koumakis G, Chen MC, Kumar S, Delinassios JG. Role of stromal fibroblasts in cancer: promoting or impeding? Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2009;30:109–120. - PubMed
-
- Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:7427–7437. - PubMed
-
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2006a;103:5947–5952. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- U54 CA163191/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- DK55001/DK/NIDDK NIH HHS/United States
- U01 CA151925/CA/NCI NIH HHS/United States
- DK81976/DK/NIDDK NIH HHS/United States
- CA163191/CA/NCI NIH HHS/United States
- R01 DK081576/DK/NIDDK NIH HHS/United States
- CA155370/CA/NCI NIH HHS/United States
- UO1 CA151925/CA/NCI NIH HHS/United States
- R01 CA125550/CA/NCI NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
- R01 CA155370/CA/NCI NIH HHS/United States
- R01 CA113669/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

