Direct observations of amyloid β self-assembly in live cells provide insights into differences in the kinetics of Aβ(1-40) and Aβ(1-42) aggregation
- PMID: 24856820
- PMCID: PMC4067742
- DOI: 10.1016/j.chembiol.2014.03.014
Direct observations of amyloid β self-assembly in live cells provide insights into differences in the kinetics of Aβ(1-40) and Aβ(1-42) aggregation
Abstract
Insight into how amyloid β (Aβ) aggregation occurs in vivo is vital for understanding the molecular pathways that underlie Alzheimer's disease and requires new techniques that provide detailed kinetic and mechanistic information. Using noninvasive fluorescence lifetime recordings, we imaged the formation of Aβ(1-40) and Aβ(1-42) aggregates in live cells. For both peptides, the cellular uptake via endocytosis is rapid and spontaneous. They are then retained in lysosomes, where their accumulation leads to aggregation. The kinetics of Aβ(1-42) aggregation are considerably faster than those of Aβ(1-40) and, unlike those of the latter peptide, show no detectable lag phase. We used superresolution fluorescence imaging to examine the resulting aggregates and could observe compact amyloid structures, likely because of spatial confinement within cellular compartments. Taken together, these findings provide clues as to how Aβ aggregation may occur within neurons.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
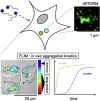
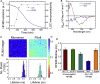
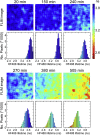


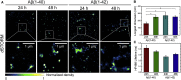
Comment in
-
Timing protein assembly in neurons.Chem Biol. 2014 Jun 19;21(6):703-4. doi: 10.1016/j.chembiol.2014.06.001. Chem Biol. 2014. PMID: 24950101
References
-
- Billings L.M., Oddo S., Green K.N., McGaugh J.L., LaFerla F.M. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer’s A4/β amyloid peptide analogs. J. Biol. Chem. 1992;267:546–554. - PubMed
-
- Burdick D., Kosmoski J., Knauer M.F., Glabe C.G. Preferential adsorption, internalization and resistance to degradation of the major isoform of the Alzheimer’s amyloid peptide, A β 1-42, in differentiated PC12 cells. Brain Res. 1997;746:275–284. - PubMed
-
- Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., Relini A., Stefani M., Dobson C.M., Cecchi C., Chiti F. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 2010;6:140–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

