Biological perspectives of delayed fracture healing
- PMID: 24857030
- PMCID: PMC4406220
- DOI: 10.1016/j.injury.2014.04.003
Biological perspectives of delayed fracture healing
Abstract
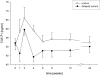
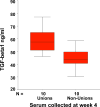
Fracture healing is a complex biological process that requires interaction among a series of different cell types. Maintaining the appropriate temporal progression and spatial pattern is essential to achieve robust healing. We can temporally assess the biological phases via gene expression, protein analysis, histologically, or non-invasively using biomarkers as well as imaging techniques. However, determining what leads to normal versus abnormal healing is more challenging. Since the ultimate outcome of fracture healing is to restore the original functions of bone, assessment of fracture healing should include not only monitoring the restoration of structure and mechanical function, but also an evaluation of the restoration of normal bone biology. Currently few non-invasive measures of biological factors of healing exist; however, recent studies that have correlated non-invasive measures with fracture healing outcome in humans have shown that serum TGFbeta1 levels appear to be an indicator of healing versus non-healing. In the future, developing additional measures to assess biological healing will improve the reliability and permit us to assess stages of fracture healing. Additionally, new functional imaging technologies could prove useful for better understanding both normal fracture healing and predicting dysfunctional healing in human patients.
Keywords: Bone healing; Mediators; Non-union; TGFbeta1; Vascularity.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop. 1998;(355 Suppl):S7–21. - PubMed
-
- Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br. 2007;89(4):425–33. - PubMed
-
- Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350(3):557–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

