Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects
- PMID: 24857658
- PMCID: PMC4057960
- DOI: 10.1016/j.celrep.2014.04.037
Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects
Abstract
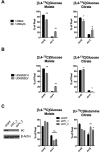
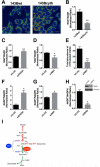
Mammalian cells generate citrate by decarboxylating pyruvate in the mitochondria to supply the tricarboxylic acid (TCA) cycle. In contrast, hypoxia and other impairments of mitochondrial function induce an alternative pathway that produces citrate by reductively carboxylating α-ketoglutarate (AKG) via NADPH-dependent isocitrate dehydrogenase (IDH). It is unknown how cells generate reducing equivalents necessary to supply reductive carboxylation in the setting of mitochondrial impairment. Here, we identified shared metabolic features in cells using reductive carboxylation. Paradoxically, reductive carboxylation was accompanied by concomitant AKG oxidation in the TCA cycle. Inhibiting AKG oxidation decreased reducing equivalent availability and suppressed reductive carboxylation. Interrupting transfer of reducing equivalents from NADH to NADPH by nicotinamide nucleotide transhydrogenase increased NADH abundance and decreased NADPH abundance while suppressing reductive carboxylation. The data demonstrate that reductive carboxylation requires bidirectional AKG metabolism along oxidative and reductive pathways, with the oxidative pathway producing reducing equivalents used to operate IDH in reverse.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- ADAM J, YANG M, BAUERSCHMIDT C, KITAGAWA M, O'FLAHERTY L, MAHESWARAN P, OZKAN G, SAHGAL N, BABAN D, KATO K, SAITO K, IINO K, IGARASHI K, STRATFORD M, PUGH C, TENNANT DA, LUDWIG C, DAVIES B, RATCLIFFE PJ, EL-BAHRAWY M, ASHRAFIAN H, SOGA T, POLLARD PJ. A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell Rep. 2013;3:1440–8. - PMC - PubMed
-
- BAYSAL BE, FERRELL RE, WILLETT-BROZICK JE, LAWRENCE EC, MYSSIOREK D, BOSCH A, VAN DER MEY A, TASCHNER PE, RUBINSTEIN WS, MYERS EN, RICHARD CW, 3RD, CORNELISSE CJ, DEVILEE P, DEVLIN B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

