The redox-Mannich reaction
- PMID: 24857691
- PMCID: PMC4059224
- DOI: 10.1021/ol501365j
The redox-Mannich reaction
Abstract
A complement to the classic three-component Mannich reaction, the redox-Mannich reaction, utilizes the same starting materials but incorporates an isomerization step that enables the facile preparation of ring-substituted β-amino ketones. Reactions occur under relatively mild conditions and are facilitated by benzoic acid.
Figures
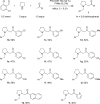
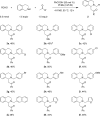

References
-
- Mannich C.; Krösche W. Arch. Pharm. 1912, 250, 647.
-
-
Selected recent reviews on the Mannich reaction:
- Arend M.; Westermann B.; Risch N. Angew. Chem., Int. Ed. 1998, 37, 1044. - PubMed
- Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541. - PMC - PubMed
- Ting A.; Schaus S. E. Eur. J. Org. Chem. 2007, 5797.
- Verkade J. M. M.; van Hemert L. J. C.; Quaedflieg P.; Rutjes F. Chem. Soc. Rev. 2008, 37, 29. - PubMed
- Arrayas R. G.; Carretero J. C. Chem. Soc. Rev. 2009, 38, 1940. - PubMed
- Kobayashi S.; Mori Y.; Fossey J. S.; Salter M. M. Chem. Rev. 2011, 111, 2626. - PubMed
- Girling P. R.; Kiyoi T.; Whiting A. Org. Biomol. Chem. 2011, 9, 3105. - PubMed
- Noble A.; Anderson J. C. Chem. Rev. 2013, 113, 2887. - PubMed
- Karimi B.; Enders D.; Jafari E. Synthesis 2013, 45, 2769.
-
-
-
Recent examples of oxidative Mannich reactions:
- Li Z.; Li C.-J. J. Am. Chem. Soc. 2005, 127, 3672. - PubMed
- Li Z.; Li C.-J. Eur. J. Org. Chem. 2005, 3173.
- Dubs C.; Hamashima Y.; Sasamoto N.; Seidel T. M.; Suzuki S.; Hashizume D.; Sodeoka M. J. Org. Chem. 2008, 73, 5859. - PubMed
- So M.-H.; Liu Y.; Ho C.-M.; Che C.-M. Chem.—Asian J. 2009, 4, 1551. - PubMed
- Shen Y.; Li M.; Wang S.; Zhan T.; Tan Z.; Guo C.-C. Chem. Commun. 2009, 953. - PubMed
- Sud A.; Sureshkumar D.; Klussmann M. Chem. Commun. 2009, 3169. - PubMed
- Shu X. Z.; Xia X. F.; Yang Y. F.; Ji K. G.; Liu X. Y.; Liang Y. M. J. Org. Chem. 2009, 74, 7464. - PubMed
- Chu L.; Zhang X.; Qing F.-L. Org. Lett. 2009, 11, 2197. - PubMed
- Grigg R.; Somasunderam A.; Sridharan V.; Keep A. Synlett 2009, 97.
- Sureshkumar D.; Sud A.; Klussmann M. Synlett 2009, 1558.
- Richter H.; García Mancheño O. Eur. J. Org. Chem. 2010, 4460.
- Shu X.-Z.; Yang Y.-F.; Xia X.-F.; Ji K.-G.; Liu X.-Y.; Liang Y.-M. Org. Biomol. Chem. 2010, 8, 4077. - PubMed
- Zeng T. Q.; Song G. H.; Moores A.; Li C. J. Synlett 2010, 2002.
- Zhang G.; Zhang Y.; Wang R. Angew. Chem., Int. Ed. 2011, 50, 10429. - PubMed
- Rueping M.; Vila C.; Koenigs R. M.; Poscharny K.; Fabry D. C. Chem. Commun. 2011, 47, 2360. - PubMed
- Pan Y.; Kee C. W.; Chen L.; Tan C.-H. Green Chem. 2011, 13, 2682.
- Boess E.; Sureshkumar D.; Sud A.; Wirtz C.; Farès C.; Klussmann M. J. Am. Chem. Soc. 2011, 133, 8106. - PubMed
- Su W. K.; Yu J. B.; Li Z. H.; Jiang Z. J. J. Org. Chem. 2011, 76, 9144. - PubMed
- Hari D. P.; Koenig B. Org. Lett. 2011, 13, 3852. - PubMed
- Xie J.; Li H.; Zhou J.; Cheng Y.; Zhu C. Angew. Chem., Int. Ed. 2012, 51, 1252. - PubMed
- Zhang J.; Tiwari B.; Xing C.; Chen X.; Chi Y. R. Angew. Chem., Int. Ed. 2012, 51, 3649. - PubMed
- Cherevatskaya M.; Neumann M.; Füldner S.; Harlander C.; Kümmel S.; Dankesreiter S.; Pfitzner A.; Zeitler K.; König B. Angew. Chem., Int. Ed. 2012, 51, 4062. - PubMed
- Liu Q.; Li Y. N.; Zhang H. H.; Chen B.; Tung C. H.; Wu L. Z. Chem.—Eur. J. 2012, 18, 620. - PubMed
- Rueping M.; Zoller J.; Fabry D. C.; Poscharny K.; Koenigs R. M.; Weirich T. E.; Mayer J. Chem.—Eur. J. 2012, 18, 3478. - PubMed
- Alagiri K.; Devadig P.; Prabhu K. R. Chem.—Eur. J. 2012, 18, 5160. - PubMed
- Boess E.; Schmitz C.; Klussmann M. J. Am. Chem. Soc. 2012, 134, 5317. - PubMed
- Freeman D. B.; Furst L.; Condie A. G.; Stephenson C. R. J. Org. Lett. 2012, 14, 94. - PMC - PubMed
- Zhao G.; Yang C.; Guo L.; Sun H.; Chen C.; Xia W. Chem. Commun. 2012, 48, 2337. - PubMed
- Möhlmann L.; Baar M.; Rieß J.; Antonietti M.; Wang X.; Blechert S. Adv. Synth. Catal. 2012, 354, 1909.
- Rueping M.; Vila C.; Bootwicha T. ACS Catal. 2013, 3, 1676.
- Zhang G.; Wang S.; Ma Y.; Kong W.; Wang R. Adv. Synth. Catal. 2013, 355, 874.
- Zhang G.; Ma Y.; Wang S.; Kong W.; Wang R. Chem. Sci. 2013, 4, 2645.
- Ratnikov M. O.; Xu X. F.; Doyle M. P. J. Am. Chem. Soc. 2013, 135, 9475. - PubMed
- Liu X.; Sun B.; Xie Z.; Qin X.; Liu L.; Lou H. J. Org. Chem. 2013, 78, 3104. - PubMed
- Xue Q. C.; Xie J.; Jin H. M.; Cheng Y. X.; Zhu C. J. Org. Biomol. Chem. 2013, 11, 1606. - PubMed
- Nobuta T.; Tada N.; Fujiya A.; Kariya A.; Miura T.; Itoh A. Org. Lett. 2013, 15, 574. - PubMed
- Dhineshkumar J.; Lamani M.; Alagiri K.; Prabhu K. R. Org. Lett. 2013, 15, 1092. - PubMed
- Bergonzini G.; Schindler C. S.; Wallentin C.-J.; Jacobsen E. N.; Stephenson C. R. J. Chem. Sci. 2014, 5, 112. - PMC - PubMed
- Tanoue A.; Yoo W.-J.; Kobayashi S. Org. Lett. 2014, 16, 2346. - PubMed
-
-
-
Selected reviews on CDC reactions:
- Li C.-J. Acc. Chem. Res. 2009, 42, 335. - PubMed
- Scheuermann C. J. Chem.—Asian J. 2010, 5, 436. - PubMed
- Yoo W. J.; Li C. J. Top. Curr. Chem. 2010, 292, 281. - PubMed
- Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. - PubMed
- Liu C.; Zhang H.; Shi W.; Lei A. W. Chem. Rev. 2011, 111, 1780. - PubMed
- Cho S. H.; Kim J. Y.; Kwak J.; Chang S. Chem. Soc. Rev. 2011, 40, 5068. - PubMed
- Klussmann M.; Sureshkumar D. Synthesis 2011, 353.
- Zhang C.; Tang C. H.; Jiao N. Chem. Soc. Rev. 2012, 41, 3464. - PubMed
- Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. - PubMed
-
-
-
Other selected reviews on amine C–H functionalization, including redox-neutral approaches:
- Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443.
- Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2625.
- Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. - PubMed
- Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem.—Eur. J. 2010, 16, 2654. - PubMed
- Wendlandt A. E.; Suess A. M.; Stahl S. S. Angew. Chem., Int. Ed. 2011, 50, 11062. - PubMed
- Sun C. L.; Li B. J.; Shi Z. J. Chem. Rev. 2011, 111, 1293. - PubMed
- Jones K. M.; Klussmann M. Synlett 2012, 23, 159.
- Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374. - PMC - PubMed
- Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem.—Eur. J. 2012, 18, 10092. - PubMed
- Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357.
- Peng B.; Maulide N. Chem.—Eur. J. 2013, 19, 13274. - PubMed
- Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551.
- Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137.
- Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

