MCT-1 expression and PTEN deficiency synergistically promote neoplastic multinucleation through the Src/p190B signaling activation
- PMID: 24858043
- PMCID: PMC4287651
- DOI: 10.1038/onc.2014.125
MCT-1 expression and PTEN deficiency synergistically promote neoplastic multinucleation through the Src/p190B signaling activation
Abstract
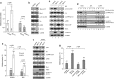
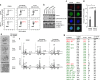
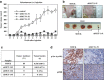
Multinucleation is associated with malignant neoplasms; however, the molecular mechanism underlying the nuclear abnormality remains unclear. Loss or mutation of PTEN promotes the development of malignant tumors. We now demonstrate that increased expression of the oncogene MCT-1 (multiple copies in T-cell malignancy 1) antagonizes PTEN gene presentation, PTEN protein stability and PTEN functional activity, thereby further promoting phosphoinositide 3 kinase/AKT signaling, survival rate and malignancies of the PTEN-deficient cells. In the PTEN-null cancer cells, MCT-1 interacts with p190B and Src in vivo, supporting that they are in proximity of the signaling complexes. MCT-1 overexpression and PTEN loss synergistically augments the Src/p190B signaling function that leads to inhibition of RhoA activity. Under such a condition, the incidence of mitotic catastrophes including spindle multipolarity and cytokinesis failure is enhanced, driving an Src/p190B/RhoA-dependent neoplastic multinucleation. Targeting MCT-1 by the short hairpin RNA markedly represses the Src/p190B function, improves nuclear structures and suppresses xenograft tumorigenicity of the PTEN-null breast cancer cells. Consistent with the oncogenic effects in vitro, clinical evidence has confirmed that MCT-1 gene stimulation is correlated with p190B gene promotion and PTEN gene suppression in human breast cancer. Accordingly, MCT-1 gene induction is recognized as a potential biomarker of breast tumor development. Abrogating MCT-1 function may be a promising stratagem for management of breast cancer involving Src hyperactivation and/or PTEN dysfunction.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

