Metabolic shift of Escherichia coli under salt stress in the presence of glycine betaine
- PMID: 24858086
- PMCID: PMC4148810
- DOI: 10.1128/AEM.00599-14
Metabolic shift of Escherichia coli under salt stress in the presence of glycine betaine
Abstract
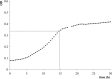
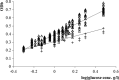
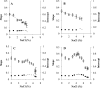
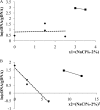
An important area of food safety focuses on bacterial survival and growth in unfavorable environments. In order to understand how bacteria adapt to stresses other than nutrient limitation in batch cultures, we need to develop mechanistic models of intracellular regulation and metabolism under stress. We studied the growth of Escherichia coli in minimal medium with added salt and different osmoprotectants. To characterize the metabolic efficiency with a robust parameter, we identified the optical density (OD) values at the inflection points of measured "OD versus time" growth curves and described them as a function of glucose concentration. We found that the metabolic efficiency parameter did not necessarily follow the trend of decreasing specific growth rate as the salt concentration increased. In the absence of osmoprotectant, or in the presence of proline, the metabolic efficiency decreased with increasing NaCl concentration. However, in the presence of choline or glycine betaine, it increased between 2 and 4.5% NaCl before declining at 5% NaCl and above. Microarray analysis of the transcriptional network and proteomics analysis with glycine betaine in the medium indicated that between 4.5 and 5% NaCl, the metabolism switched from aerobic to fermentative pathways and that the response to osmotic stress is similar to that for oxidative stress. We conclude that, although the growth rate appeared to decrease smoothly with increasing NaCl, the metabolic strategy of cells changed abruptly at a threshold concentration of NaCl.
Figures


References
-
- Feist AM, Henry CS, Reed JL, Krummenacker M, Joyce AR, Karp PD, Broadbelt LJ, Hatzimanikatis V, Palsson BO. 2007. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol. Syst. Biol. 3:121. 10.1038/msb4100155 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

