Role of oligomeric proanthocyanidins derived from an extract of persimmon fruits in the oxidative stress-related aging process
- PMID: 24858102
- PMCID: PMC6271875
- DOI: 10.3390/molecules19056707
Role of oligomeric proanthocyanidins derived from an extract of persimmon fruits in the oxidative stress-related aging process
Abstract
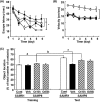
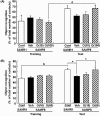
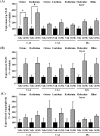
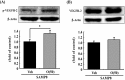
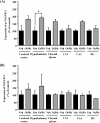
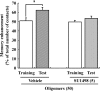
Many researchers have focused on the oligomeric form of proanthocyanidins with a lower level of polymerization found in foodstuffs such as grape seeds and blackberries. The present study indicated that the oral administration of oligomers isolated from persimmon fruits extended the lifespan of senescence-accelerated mouse prone/8 (SAMP8), a murine model of accelerated senescence. On the other hand, oligomer-treated SAMP8 did not show stereotypical behavior. We also revealed that the oral administration of oligomers improved spatial and object recognition memory in SAMP8. The density of axons in the hippocampal CA1 was significantly increased by oligomer administration. Moreover, the administration of oligomers increased the phosphorylation of vascular endothelial growth factor receptor (VEGFR)-2 in the hippocampal CA3, hypothalamus, and choroid plexus. We speculate that memory improvement accompanied by histological changes may be induced directly in the hippocampus and indirectly in the hypothalamus and choroid plexus through VEGFR-2 signaling. In the present study, we elucidated the protective effect of oligomers against memory impairment with aging. VEGFR-2 signaling may provide a new insight into ways to protect against memory deficit in the aging brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Oligomeric proanthocyanidins improve memory and enhance phosphorylation of vascular endothelial growth factor receptor-2 in senescence-accelerated mouse prone/8.Br J Nutr. 2010 Feb;103(4):479-89. doi: 10.1017/S0007114509992005. Epub 2009 Oct 13. Br J Nutr. 2010. PMID: 19822031
-
Anti-aging effects of oligomeric proanthocyanidins isolated from persimmon fruits.Drug Discov Ther. 2011 Jun;5(3):109-18. doi: 10.5582/ddt.2011.v5.3.109. Drug Discov Ther. 2011. PMID: 22466239
-
Persimmon oligomeric proanthocyanidins extend life span of senescence-accelerated mice.J Med Food. 2009 Dec;12(6):1199-205. doi: 10.1089/jmf.2009.0113. J Med Food. 2009. PMID: 20041772
-
Activity and potential mechanisms of action of persimmon tannins according to their structures: A review.Int J Biol Macromol. 2023 Jul 1;242(Pt 3):125120. doi: 10.1016/j.ijbiomac.2023.125120. Epub 2023 May 30. Int J Biol Macromol. 2023. PMID: 37263329 Review.
-
Proanthocyanidins of Natural Origin: Molecular Mechanisms and Implications for Lipid Disorder and Aging-Associated Diseases.Adv Nutr. 2019 May 1;10(3):464-478. doi: 10.1093/advances/nmy118. Adv Nutr. 2019. PMID: 30926997 Free PMC article. Review.
Cited by
-
Oligomeric Proanthocyanidins and Bamboo Leaf Flavonoids Improve the Quality of Bull Semen Cryopreservation.Molecules. 2022 Feb 8;27(3):1144. doi: 10.3390/molecules27031144. Molecules. 2022. PMID: 35164407 Free PMC article.
-
Diospyros kaki and Citrus unshiu Mixture Improves Disorders of Lipid Metabolism in Nonalcoholic Fatty Liver Disease.Can J Gastroenterol Hepatol. 2020 Dec 24;2020:8812634. doi: 10.1155/2020/8812634. eCollection 2020. Can J Gastroenterol Hepatol. 2020. PMID: 33425805 Free PMC article.
-
Effects of Grape Skin Extract on Age-Related Mitochondrial Dysfunction, Memory and Life Span in C57BL/6J Mice.Neuromolecular Med. 2016 Sep;18(3):378-95. doi: 10.1007/s12017-016-8428-4. Epub 2016 Jul 25. Neuromolecular Med. 2016. PMID: 27455862
-
The Lotus corniculatus MYB5 functions as a master regulator in proanthocyanidin biosynthesis and bioengineering.Plant Cell Rep. 2024 Nov 19;43(12):284. doi: 10.1007/s00299-024-03313-9. Plant Cell Rep. 2024. PMID: 39557697
-
Immature Persimmon Suppresses Amyloid Beta (Aβ) Mediated Cognitive Dysfunction via Tau Pathology in ICR Mice.Curr Issues Mol Biol. 2021 Jun 21;43(1):405-422. doi: 10.3390/cimb43010033. Curr Issues Mol Biol. 2021. PMID: 34205542 Free PMC article.
References
-
- Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

