A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors
- PMID: 24858213
- PMCID: PMC4059966
- DOI: 10.1007/s00401-014-1288-9
A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors
Abstract
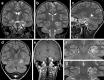
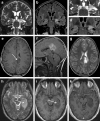
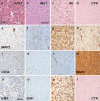
Every fourth patient submitted to epilepsy surgery suffers from a brain tumor. Microscopically, these neoplasms present with a wide-ranging spectrum of glial or glio-neuronal tumor subtypes. Gangliogliomas (GG) and dysembryoplastic neuroepithelial tumors (DNTs) are the most frequently recognized entities accounting for 65 % of 1,551 tumors collected at the European Epilepsy Brain Bank (n = 5,842 epilepsy surgery samples). These tumors often present with early seizure onset at a mean age of 16.5 years, with 77 % of neoplasms affecting the temporal lobe. Relapse and malignant progression are rare events in this particular group of brain tumors. Surgical resection should be regarded, therefore, also as important treatment strategy to prevent epilepsy progression as well as seizure- and medication-related comorbidities. The characteristic clinical presentation and broad histopathological spectrum of these highly epileptogenic brain tumors will herein be classified as "long-term epilepsy associated tumors-LEATs". LEATs differ from most other brain tumors by early onset of spontaneous seizures, and conceptually are regarded as developmental tumors to explain their pleomorphic microscopic appearance and frequent association with Focal Cortical Dysplasia Type IIIb. However, the broad neuropathologic spectrum and lack of reliable histopathological signatures make these tumors difficult to classify using the WHO system of brain tumors. As another consequence from poor agreement in published LEAT series, molecular diagnostic data remain ambiguous. Availability of surgical tissue specimens from patients which have been well characterized during their presurgical evaluation should open the possibility to systematically address the origin and epileptogenicity of LEATs, and will be further discussed herein. As a conclusion, the authors propose a novel A-B-C terminology of epileptogenic brain tumors ("epileptomas") which hopefully promote the discussion between neuropathologists, neurooncologists and epileptologists. It must be our future mission to achieve international consensus for the clinico-pathological classification of LEATs that would also involve World Health Organization (WHO) and the International League against Epilepsy (ILAE).
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

