Potassium 2-(1-hydroxypentyl)-benzoate promotes long-term potentiation in Aβ1-42-injected rats and APP/PS1 transgenic mice
- PMID: 24858312
- PMCID: PMC4088280
- DOI: 10.1038/aps.2014.29
Potassium 2-(1-hydroxypentyl)-benzoate promotes long-term potentiation in Aβ1-42-injected rats and APP/PS1 transgenic mice
Abstract
Aim: Potassium 2-(1-hydroxypentyl)-benzoate (dl-PHPB) is a new drug candidate for ischemic stroke. The aim of this study was to investigate the effects of dl-PHPB on memory deficits and long-term potentiation (LTP) impairment in animal models of Alzheimer's disease.
Methods: The expression of NMDA receptor subunits GluN1 and GluN2B in the hippocampus and cortex of APP/PS1 transgenic mice were detected using Western blot analysis. Memory deficits of the mice were evaluated with the passive avoidance test. LTP impairment was studied in the dentate region of Aβ1-42-injected rats and APP/PS1 transgenic mice.
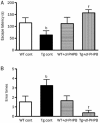
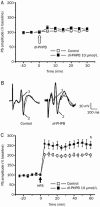
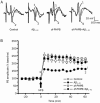
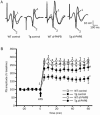
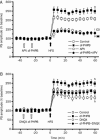
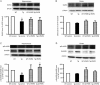
Results: APP/PS1 transgenic mice showed significantly lower levels of GluN1 and p-GluN2B in hippocampus, and chronic administration of dl-PHPB (100 mg · kg(-1) · d(-1), po) reversed the downregulation of p-GluN2B, but did not change GluN1 level in the hippocampus. Furthermore, chronic administration of dl-PHPB reversed the memory deficits in APP/PS1 transgenic mice. In the dentate region of normal rats, injection of dl-PHPB (100 μmol/L, icv) did not change the basal synaptic transmission, but significantly enhanced the high-frequency stimulation (HFS)-induced LTP, which was completely prevented by pre-injection of APV (150 μmol/L, icv). Chronic administration of dl-PHPB (100 mg · kg(-1) · d(-1), po) reversed LTP impairment in Aβ1-42-injected normal rats and APP/PS1 transgenic mice.
Conclusion: Chronic administration of dl-PHPB improves learning and memory and promotes LTP in the animal models of Alzheimer's disease, possibly via increasing p-GluN2B expression in the hippocampus.
Figures
Similar articles
-
Potassium 2-(1-hydroxypentyl)-benzoate improves memory deficits and attenuates amyloid and τ pathologies in a mouse model of Alzheimer's disease.J Pharmacol Exp Ther. 2014 Aug;350(2):361-74. doi: 10.1124/jpet.114.213140. Epub 2014 Jun 3. J Pharmacol Exp Ther. 2014. PMID: 24893984
-
Protective effect of potassium 2-(l-hydroxypentyl)-benzoate on hippocampal neurons, synapses and dystrophic axons in APP/PS1 mice.Psychopharmacology (Berl). 2019 Sep;236(9):2761-2771. doi: 10.1007/s00213-019-05251-x. Epub 2019 Jun 4. Psychopharmacology (Berl). 2019. PMID: 31165206
-
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease.Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. Neuropharmacology. 2014. PMID: 23973293
-
CART modulates beta-amyloid metabolism-associated enzymes and attenuates memory deficits in APP/PS1 mice.Neurol Res. 2017 Oct;39(10):885-894. doi: 10.1080/01616412.2017.1348689. Epub 2017 Jul 25. Neurol Res. 2017. PMID: 28743230
-
Gallic acid disruption of Aβ1-42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse.Neurobiol Dis. 2019 Apr;124:67-80. doi: 10.1016/j.nbd.2018.11.009. Epub 2018 Nov 14. Neurobiol Dis. 2019. PMID: 30447302
Cited by
-
L-3-n-butylphthalide Rescues Hippocampal Synaptic Failure and Attenuates Neuropathology in Aged APP/PS1 Mouse Model of Alzheimer's Disease.CNS Neurosci Ther. 2016 Dec;22(12):979-987. doi: 10.1111/cns.12594. Epub 2016 Jul 20. CNS Neurosci Ther. 2016. PMID: 27439966 Free PMC article.
-
Potassium 2-(l-hydroxypentyl)-benzoate attenuates neuroinflammatory responses and upregulates heme oxygenase-1 in systemic lipopolysaccharide-induced inflammation in mice.Acta Pharm Sin B. 2017 Jul;7(4):470-478. doi: 10.1016/j.apsb.2017.04.007. Epub 2017 May 9. Acta Pharm Sin B. 2017. PMID: 28752032 Free PMC article.
-
Characterization of AD-like phenotype in aged APPSwe/PS1dE9 mice.Age (Dordr). 2016 Aug;38(4):303-322. doi: 10.1007/s11357-016-9929-7. Epub 2016 Jul 21. Age (Dordr). 2016. PMID: 27439903 Free PMC article.
-
Parishin C's prevention of Aβ 1-42-induced inhibition of long-term potentiation is related to NMDA receptors.Acta Pharm Sin B. 2016 May;6(3):189-97. doi: 10.1016/j.apsb.2016.03.009. Epub 2016 Apr 22. Acta Pharm Sin B. 2016. PMID: 27175329 Free PMC article.
-
Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1-42.Int J Mol Sci. 2022 Jan 21;23(3):1193. doi: 10.3390/ijms23031193. Int J Mol Sci. 2022. PMID: 35163115 Free PMC article.
References
-
- Zhang Y, Wang L, Li J, Wang XL. 2-(1-Hydroxypentyl)-benzoate increases cerebral blood flow and reduces infarct volume in rats model of transient focal cerebral ischemia. J Pharmacol Exp Ther. 2006;317:973–9. - PubMed
-
- Zhao W, Xu S, Peng Y, Ji X, Cao D, Li J. Potassium 2-(1-hydroxypentyl)-benzoate improves learning and memory deficits in chronic cerebral hypoperfused rats. Neurosci Lett. 2013;541:155–60. - PubMed
-
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. - PubMed
-
- Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–7. - PubMed
-
- Freir DB, Holscher C, Herron CE. Blockade of long-term potentiation by beta-amyloid peptides in the CA1 region of the rat hippocampus in vivo. J Neurophysiol. 2001;85:708–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

