TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions
- PMID: 24858416
- PMCID: PMC4234683
- DOI: 10.1007/s00018-014-1647-7
TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions
Abstract
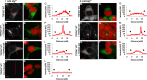
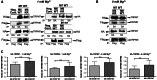
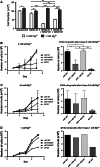
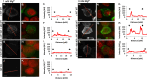
The channel kinases TRPM6 and TRPM7 are both members of the melastatin-related transient receptor potential (TRPM) subfamily of ion channels and the only known fusions of an ion channel pore with a kinase domain. TRPM6 and TRPM7 form functional, tetrameric channel complexes at the plasma membrane by heteromerization. TRPM6 was previously shown to cross-phosphorylate TRPM7 on threonine residues, but not vice versa. Genetic studies demonstrated that TRPM6 and TRPM7 fulfill non-redundant functions and that each channel contributes uniquely to the regulation of Mg(2+) homeostasis. Although there are indications that TRPM6 and TRPM7 can influence each other's cellular distribution and activity, little is known about the functional relationship between these two channel-kinases. In the present study, we examined how TRPM6 kinase activity influences TRPM7 serine phosphorylation, intracellular trafficking, and cell surface expression of TRPM7, as well as Mg(2+)-dependent cellular growth. We found TRPM7 serine phosphorylation via the TRPM6 kinase, but no TRPM6 serine phosphorylation via the TRPM7 kinase. Intracellular trafficking of TRPM7 was altered in HEK-293 epithelial kidney cells and DT40 B cells in the presence of TRPM6 with intact kinase activity, independently of the availability of extracellular Mg(2+), but TRPM6/7 surface labeling experiments indicate comparable levels of the TRPM6/7 channels at the plasma membrane. Furthermore, using a complementation approach in TRPM7-deficient DT40 B-cells, we demonstrated that wild-type TRPM6 inhibited cell growth under hypomagnesic cell culture conditions in cells co-expressing TRPM6 and TRPM7; however, co-expression of a TRPM6 kinase dead mutant had no effect-a similar phenotype was also observed in TRPM6/7 co-expressing HEK-293 cells. Our results provide first clues about how heteromer formation between TRPM6 and TRPM7 influences the biological activity of these ion channels. We show that TRPM6 regulates TRPM7 intracellular trafficking and TRPM7-dependent cell growth. All these effects are dependent upon the presence of an active TRPM6 kinase domain. Dysregulated Mg(2+)-homeostasis causes or exacerbates many pathologies. As TRPM6 and TRPM7 are expressed simultaneously in numerous cell types, understanding how their relationship impacts regulation of Mg(2+)-uptake is thus important knowledge.
Figures

Similar articles
-
The channel kinases TRPM6 and TRPM7 are functionally nonredundant.J Biol Chem. 2005 Nov 11;280(45):37763-71. doi: 10.1074/jbc.M509175200. Epub 2005 Sep 2. J Biol Chem. 2005. PMID: 16150690
-
The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels.J Biol Chem. 2014 Feb 21;289(8):5217-27. doi: 10.1074/jbc.M113.512285. Epub 2014 Jan 2. J Biol Chem. 2014. PMID: 24385424 Free PMC article.
-
Role of the alpha-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP.J Biol Chem. 2008 Jul 18;283(29):19999-20007. doi: 10.1074/jbc.M800167200. Epub 2008 May 19. J Biol Chem. 2008. PMID: 18490453
-
The Different Roles of The Channel-Kinases TRPM6 and TRPM7.Curr Med Chem. 2015;22(25):2943-53. doi: 10.2174/0929867322666150716115644. Curr Med Chem. 2015. PMID: 26179995 Review.
-
Role of kinase-coupled TRP channels in mineral homeostasis.Pharmacol Ther. 2018 Apr;184:159-176. doi: 10.1016/j.pharmthera.2017.11.003. Epub 2017 Nov 9. Pharmacol Ther. 2018. PMID: 29129644 Review.
Cited by
-
Flavaglines Stimulate Transient Receptor Potential Melastatin Type 6 (TRPM6) Channel Activity.PLoS One. 2015 Mar 16;10(3):e0119028. doi: 10.1371/journal.pone.0119028. eCollection 2015. PLoS One. 2015. PMID: 25774985 Free PMC article.
-
A distal convoluted tubule-specific isoform of murine SLC41A3 extrudes magnesium.Acta Physiol (Oxf). 2025 Mar;241(3):e70018. doi: 10.1111/apha.70018. Acta Physiol (Oxf). 2025. PMID: 39931759 Free PMC article.
-
TRPM7 and its role in neurodegenerative diseases.Channels (Austin). 2015;9(5):253-61. doi: 10.1080/19336950.2015.1075675. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218331 Free PMC article. Review.
-
What is the evidence for the role of TRP channels in inflammatory and immune cells?Br J Pharmacol. 2016 Mar;173(6):953-69. doi: 10.1111/bph.13392. Epub 2016 Feb 18. Br J Pharmacol. 2016. PMID: 26603538 Free PMC article. Review.
-
Novel Alleles of gon-2, a C. elegans Ortholog of Mammalian TRPM6 and TRPM7, Obtained by Genetic Reversion Screens.PLoS One. 2015 Nov 25;10(11):e0143445. doi: 10.1371/journal.pone.0143445. eCollection 2015. PLoS One. 2015. PMID: 26606136 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

