Interferon alpha plus 13-cis-retinoic acid modulation of BCL-2 plus paclitaxel for recurrent small-cell lung cancer (SCLC): an Eastern Cooperative Oncology Group study (E6501)
- PMID: 24858462
- PMCID: PMC4296897
- DOI: 10.1007/s00280-014-2427-7
Interferon alpha plus 13-cis-retinoic acid modulation of BCL-2 plus paclitaxel for recurrent small-cell lung cancer (SCLC): an Eastern Cooperative Oncology Group study (E6501)
Abstract
Background: Patients with recurrent small-cell lung cancer (SCLC) have dismal outcomes. The failure of salvage therapy is due to the possible development of resistance mechanisms, such as the upregulation of the anti-apoptosis protein, Bcl-2. We conducted a phase II study to evaluate if modulation of Bcl-2 with 13-cis-retinoic acid (13-CRA) and interferon alpha could improve response rates when combined with paclitaxel in patients with recurrent SCLC.
Methods: Patients with recurrent SCLC and measurable disease were treated with interferon alpha at 6 million units/m² subcutaneously and 13-CRA 1 mg/kg orally on days 1 and 2 and paclitaxel 75 mg/m² intravenously on day 2 of each week for 6 weeks of an 8-week treatment cycle. Treatment was continued until disease progression, development of unacceptable toxicity, or withdrawal of consent. The primary endpoint was response rate with secondary endpoints of progression-free survival (PFS) and overall survival (OS). Bcl-2 levels were assessed in peripheral blood mononuclear cells (PBMCs).
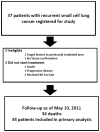
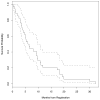
Results: Thirty-seven patients were enrolled; 34 were included in the intention-to-treat analysis as 3 patients were ineligible for the study. There were 3 partial responses (9 %), and 5 patients had stable disease (15 %) as best response. The median PFS was 2 months, and median OS was 6.2 months. Although mean Bcl-2 protein levels decreased with therapy in PBMCs, there was no association between Bcl-2 levels and response rate or survival.
Conclusion: Despite sound pre-clinical evidence, the addition of 13-CRA and interferon alpha to paclitaxel did not improve outcomes for recurrent SCLC.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–44. - PubMed
-
- Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526–7. - PubMed
-
- Abrams J, Doyle LA, Aisner J. Staging, prognostic factors, and special considerations in small cell lung cancer. Semin Oncol. 1988;15(3):261–77. - PubMed
-
- Aisner J, Alberto P, Bitran J, Comis R, Daniels J, Hansen H, et al. Role of chemotherapy in small cell lung cancer: a consensus report of the International Association for the Study of Lung Cancer workshop. Cancer Treat Rep. 1983;67(1):37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA21076/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA107868/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA086802/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180870/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources