HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling
- PMID: 24858848
- PMCID: PMC4101640
- DOI: 10.1152/ajpheart.00149.2014
HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling
Abstract
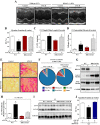
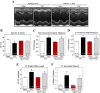
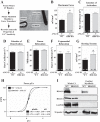
Little is known about the function of the cytoplasmic histone deacetylase HDAC6 in striated muscle. Here, we addressed the role of HDAC6 in cardiac and skeletal muscle remodeling induced by the peptide hormone angiotensin II (ANG II), which plays a central role in blood pressure control, heart failure, and associated skeletal muscle wasting. Comparable with wild-type (WT) mice, HDAC6 null mice developed cardiac hypertrophy and fibrosis in response to ANG II. However, whereas WT mice developed systolic dysfunction upon treatment with ANG II, cardiac function was maintained in HDAC6 null mice treated with ANG II for up to 8 wk. The cardioprotective effect of HDAC6 deletion was mimicked in WT mice treated with the small molecule HDAC6 inhibitor tubastatin A. HDAC6 null mice also exhibited improved left ventricular function in the setting of pressure overload mediated by transverse aortic constriction. HDAC6 inhibition appeared to preserve systolic function, in part, by enhancing cooperativity of myofibrillar force generation. Finally, we show that HDAC6 null mice are resistant to skeletal muscle wasting mediated by chronic ANG-II signaling. These findings define novel roles for HDAC6 in striated muscle and suggest potential for HDAC6-selective inhibitors for the treatment of cardiac dysfunction and muscle wasting in patients with heart failure.
Keywords: cardiac dysfunction; deacetylase; muscle atrophy.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Cabello-Verrugio C, Cordova G, Salas JD. Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci 13: 560–569, 2012 - PubMed
-
- Cohen TJ, Waddell DS, Barrientos T, Lu Z, Feng G, Cox GA, Bodine SC, Yao TP. The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. J Biol Chem 282: 33752–33759, 2007 - PubMed
-
- Di Bari M, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, Williamson JD, Marchionni N, Pahor M. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc 52: 961–966, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- TL5TL1-RR-025778-04/RR/NCRR NIH HHS/United States
- R01 HL116848/HL/NHLBI NIH HHS/United States
- T32 HL007822/HL/NHLBI NIH HHS/United States
- R01 EY021502/EY/NEI NIH HHS/United States
- R21 AG043822/AG/NIA NIH HHS/United States
- TL1 TR001081/TR/NCATS NIH HHS/United States
- 5T32-HL-007822-12/HL/NHLBI NIH HHS/United States
- T32 GM007635/GM/NIGMS NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- HL-116848/HL/NHLBI NIH HHS/United States
- R01 EY024986/EY/NEI NIH HHS/United States
- AG-043822/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

