X inactivation plays a major role in the gender bias in somatic expansion in a mouse model of the fragile X-related disorders: implications for the mechanism of repeat expansion
- PMID: 24858908
- PMCID: PMC4140472
- DOI: 10.1093/hmg/ddu213
X inactivation plays a major role in the gender bias in somatic expansion in a mouse model of the fragile X-related disorders: implications for the mechanism of repeat expansion
Abstract
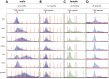
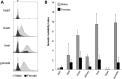
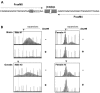
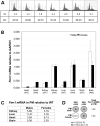
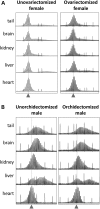
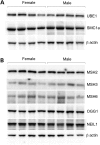
The Fragile X-related disorders are X-linked disorders resulting from the inheritance of FMR1 alleles with >54 CGG/CCG repeats in their 5' UTR. The repeats expand both somatically and on intergenerational transmission and increased repeat numbers are associated with increased risk of disease and increased risk of further expansion. The mechanism responsible for expansion is unknown. Here, we show in a knockin mouse model of these disorders that somatic expansion is much less common in females than in males. We show that this is due in large part to the fact that expansions occur only when the repeat is on the active X chromosome. However, even when this is taken into account, expansions in females are still less common than expected. This additional gender effect is not due to a protective effect of estrogen, a deleterious effect of testosterone or to differences in the expression of the Fmr1 gene or a variety of X-linked and autosomal DNA repair genes. However, our data do suggest that a higher level of expression of genes that protect against oxidative damage in females may contribute to their lower levels of expansion. Whatever the basis, our data suggest that the risk for somatic expansion may be lower in women than it is in men. This could help explain the reduced penetrance of some aspects of disease pathology in women. The fact that expansion only occurs when the Fmr1 allele is on the active X chromosome has important implications for the mechanism of repeat expansion.
Published by Oxford University Press 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
References
-
- Sherman S.L. Premature ovarian failure in the fragile X syndrome. Am. J. Med. Genet. 2000;97:189–194. - PubMed
-
- Fry M., Usdin K. Human Nucleotide Expansion Disorders. Springer, Heidelberg; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

