Indian Hedgehog mediates gastrin-induced proliferation in stomach of adult mice
- PMID: 24859162
- PMCID: PMC4211430
- DOI: 10.1053/j.gastro.2014.05.006
Indian Hedgehog mediates gastrin-induced proliferation in stomach of adult mice
Abstract
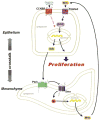
Background & aims: Loss of expression of Sonic Hedgehog (Shh) from parietal cells results in hypergastrinemia in mice, accompanied by increased expression of Indian Hedgehog (Ihh) and hyperproliferation of surface mucous cells. We investigated whether hypergastrinemia induces gastric epithelial proliferation by activating Ihh signaling in mice.
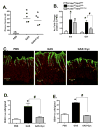
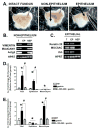
Methods: We studied mice with parietal cell-specific deletion of Shh (PC-Shh(KO)) and hypergastrinemia, crossed with gastrin-deficient (GKO) mice (PC-Shh(KO)/GKO). When mice were 3-4 months old, gastric tissues were collected and analyzed by histology, for incorporation of bromodeoxyuridine, and for expression of the surface mucous cell marker Ulex europaeus. PC-Shh(KO)/GKO mice were given gastrin infusions for 7 days; gastric surface epithelium was collected and expression of Ihh was quantified by laser capture microdissection followed by quantitative reverse transcriptase polymerase chain reaction. Mouse stomach-derived organoids were incubated with or without inhibitors of WNT (DKK1) or Smoothened (vismodegib) and then cocultured with immortalized stomach mesenchymal cells, to assess proliferative responses to gastrin.
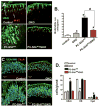
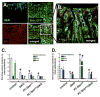
Results: Gastric tissues from PC-Shh(KO)/GKO mice with hypergastrinemia had an expanded surface pit epithelium, indicated by a significant increase in numbers of bromodeoxyuridine- and Ulex europaeus-positive cells, but there was no evidence for hyperproliferation. Gastrin infusion of PC PC-Shh(KO)/GKO mice increased expression of Ihh and proliferation within the surface epithelium compared with mice given infusions of saline. In gastric organoids cocultured with immortalized stomach mesenchymal cells, antagonists of WNT and Smoothened inhibited gastrin-induced proliferation and WNT activity. Activity of WNT in media collected from immortalized stomach mesenchymal cells correlated with increased expression of glioma-associated oncogene homolog 1, and was inhibited by DKK1 or vismodegib.
Conclusions: Ihh signaling mediates gastrin-induced proliferation of epithelial cells in stomachs of adult mice.
Keywords: Development; Gastric Epithelium; Signal Transduction; Tissue Regeneration.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells.Gastroenterology. 2010 Feb;138(2):550-61, 561.e1-8. doi: 10.1053/j.gastro.2009.11.002. Epub 2009 Nov 10. Gastroenterology. 2010. PMID: 19909751 Free PMC article.
-
Mesenchymal stem cells induce epithelial proliferation within the inflamed stomach.Am J Physiol Gastrointest Liver Physiol. 2014 Jun 15;306(12):G1075-88. doi: 10.1152/ajpgi.00489.2012. Epub 2014 May 1. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24789207 Free PMC article.
-
Sonic Hedgehog contributes to gastric mucosal restitution after injury.Lab Invest. 2013 Jan;93(1):96-111. doi: 10.1038/labinvest.2012.148. Epub 2012 Oct 22. Lab Invest. 2013. PMID: 23090636 Free PMC article.
-
Hedgehog signaling in the stomach.Curr Opin Pharmacol. 2016 Dec;31:76-82. doi: 10.1016/j.coph.2016.09.003. Epub 2016 Oct 14. Curr Opin Pharmacol. 2016. PMID: 27750091 Free PMC article. Review.
-
Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review).Int J Mol Med. 2006 Dec;18(6):1019-23. Int J Mol Med. 2006. PMID: 17089004 Review.
Cited by
-
CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation.PLoS Pathog. 2015 Feb 6;11(2):e1004663. doi: 10.1371/journal.ppat.1004663. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25658601 Free PMC article.
-
Development of Human Pituitary Neuroendocrine Tumor Organoids to Facilitate Effective Targeted Treatments of Cushing's Disease.Cells. 2022 Oct 23;11(21):3344. doi: 10.3390/cells11213344. Cells. 2022. PMID: 36359740 Free PMC article.
-
Gastrin induces parathyroid hormone-like hormone expression in gastric parietal cells.Am J Physiol Gastrointest Liver Physiol. 2017 Jun 1;312(6):G649-G657. doi: 10.1152/ajpgi.00366.2016. Epub 2017 Apr 13. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28408643 Free PMC article.
-
Generation of 3D human gastrointestinal organoids: principle and applications.Cell Regen. 2020 Jun 10;9(1):6. doi: 10.1186/s13619-020-00040-w. Cell Regen. 2020. PMID: 32588198 Free PMC article. Review.
-
Role of metaplasia during gastric regeneration.Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C947-C954. doi: 10.1152/ajpcell.00415.2019. Epub 2020 Aug 5. Am J Physiol Cell Physiol. 2020. PMID: 32755448 Free PMC article. Review.
References
-
- Van Den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. - PubMed
-
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. - PubMed
-
- Kang DH, Han ME, Song MH, Lee YS, Kim EH, Kim HJ, Kim GH, Kim DH, Yoon S, Baek SY, Kim BS, Kim JB, Oh SO. The role of hedgehog signaling during gastric regeneration. J Gastroenterol. 2009;44:372–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

