Prolactin induces apoptosis of lactotropes in female rodents
- PMID: 24859278
- PMCID: PMC4032245
- DOI: 10.1371/journal.pone.0097383
Prolactin induces apoptosis of lactotropes in female rodents
Abstract
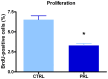
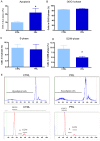
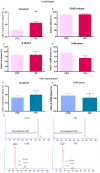
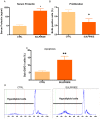
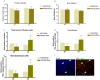
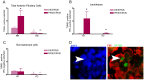
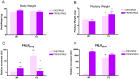
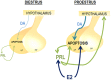
Anterior pituitary cell turnover occurring during female sexual cycle is a poorly understood process that involves complex regulation of cell proliferation and apoptosis by multiple hormones. In rats, the prolactin (PRL) surge that occurs at proestrus coincides with the highest apoptotic rate. Since anterior pituitary cells express the prolactin receptor (PRLR), we aimed to address the actual role of PRL in the regulation of pituitary cell turnover in cycling females. We showed that acute hyperprolactinemia induced in ovariectomized rats using PRL injection or dopamine antagonist treatment rapidly increased apoptosis and decreased proliferation specifically of PRL producing cells (lactotropes), suggesting a direct regulation of these cell responses by PRL. To demonstrate that apoptosis naturally occurring at proestrus was regulated by transient elevation of endogenous PRL levels, we used PRLR-deficient female mice (PRLRKO) in which PRL signaling is totally abolished. According to our hypothesis, no increase in lactotrope apoptotic rate was observed at proestrus, which likely contributes to pituitary tumorigenesis observed in these animals. To decipher the molecular mechanisms underlying PRL effects, we explored the isoform-specific pattern of PRLR expression in cycling wild type females. This analysis revealed dramatic changes of long versus short PRLR ratio during the estrous cycle, which is particularly relevant since these isoforms exhibit distinct signaling properties. This pattern was markedly altered in a model of chronic PRLR signaling blockade involving transgenic mice expressing a pure PRLR antagonist (TGΔ1-9-G129R-hPRL), providing evidence that PRL regulates the expression of its own receptor in an isoform-specific manner. Taken together, these results demonstrate that i) the PRL surge occurring during proestrus is a major proapoptotic signal for lactotropes, and ii) partial or total deficiencies in PRLR signaling in the anterior pituitary may result in pituitary hyperplasia and eventual prolactinoma development, as observed in TGΔ1-9-G129R-hPRL and PRLRKO mice, respectively.
Conflict of interest statement
Figures
References
-
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19: 225–268. - PubMed
-
- Fernandez I, Touraine P, Goffin V (2010) Prolactin and human tumourogenesis. J Neuroendocrinol 22: 771–777. - PubMed
-
- Bernichtein S, Touraine P, Goffin V (2010) New concepts in prolactin biology. J Endocrinol 206: 1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

