Early development of broadly neutralizing antibodies in HIV-1-infected infants
- PMID: 24859529
- PMCID: PMC4060046
- DOI: 10.1038/nm.3565
Early development of broadly neutralizing antibodies in HIV-1-infected infants
Abstract
Eliciting protective neutralizing antibodies (NAbs) against HIV-1 is daunting because of the extensive genetic and antigenic diversity of HIV-1. Moreover, broad and potent responses are uncommon even during persistent infection, with only a subset of adults developing broadly neutralizing antibodies (bNAbs) that recognize viral variants from different HIV-1 clades. It is not known whether bNAbs can also arise in HIV-1-infected infants, who typically progress to disease faster than adults, presumably in part due to an immature immune system. Here, we show that bNAbs develop at least as commonly in infants as in adults. Cross-clade NAb responses were detected in 20/28 infected infants, in some cases within 1 year of infection. Among infants with breadth of responses within the top quartile, neutralization of tier 2 or 3 variants from multiple clades was detected at 20 months after infection. These findings suggest that, even in early life, there is sufficient B cell functionality to mount bNAbs against HIV-1. Additionally, the relatively early appearance of bNAbs in infants may provide a unique setting for understanding the pathways of B cell maturation leading to bNAbs.
Conflict of interest statement
L.G. designed and performed experiments, analyzed data, and wrote the manuscript. V.C. performed experiments. R.N. oversaw the cohort and clinical aspects. J.O. conceived and oversaw the study, and edited the manuscript.
Figures

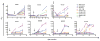

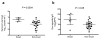
Comment in
-
Lessons from babies: inducing HIV-1 broadly neutralizing antibodies.Nat Med. 2014 Jun;20(6):583-5. doi: 10.1038/nm.3598. Nat Med. 2014. PMID: 24901564 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

