Transcranial cavitation detection in primates during blood-brain barrier opening--a performance assessment study
- PMID: 24859660
- PMCID: PMC4034133
- DOI: 10.1109/TUFFC.2014.2992
Transcranial cavitation detection in primates during blood-brain barrier opening--a performance assessment study
Abstract
Focused ultrasound (FUS) has been shown promise in treating the brain locally and noninvasively. Transcranial passive cavitation detection (PCD) provides methodology for monitoring the treatment in real time, but the skull effects remain a major challenge for its translation to the clinic. In this study, we investigated the sensitivity, reliability, and limitations of PCD through primate (macaque and human) skulls in vitro. The results were further correlated with the in vivo macaque studies including the transcranial PCD calibration and real-time monitoring of blood-brain barrier (BBB) opening, with magnetic resonance imaging assessing the opening and safety. The stable cavitation doses using harmonics (SCDh) and ultraharmonics (SCDu), the inertial cavitation dose (ICD), and the cavitation SNR were quantified based on the PCD signals. Results showed that through the macaque skull, the pressure threshold for detecting the SCDh remained the same as without the skull in place, whereas it increased for the SCDu and ICD; through the human skull, it increased for all cavitation doses. The transcranial PCD was found to be reliable both in vitro and in vivo when the transcranial cavitation SNR exceeded the 1-dB detection limit through the in vitro macaque (attenuation: 4.92 dB/mm) and human (attenuation: 7.33 dB/ mm) skull. In addition, using long pulses enabled reliable PCD monitoring and facilitate BBB opening at low pressures. The in vivo results showed that the SCDh became detectable at pressures as low as 100 kPa; the ICD became detectable at 250 kPa, although it could occur at lower pressures; and the SCDu became detectable at 700 kPa and was less reliable at lower pressures. Real-time monitoring of PCD was further implemented during BBB opening, with successful and safe opening achieved at 250 to 600 kPa in both the thalamus and the putamen. In conclusion, this study shows that transcranial PCD in macaques in vitro and in vivo, and in humans in vitro, is reliable by improving the cavitation SNR beyond the 1-dB detection limit.
Figures

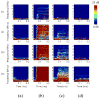




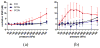
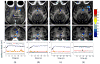
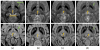
Similar articles
-
In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice.Phys Med Biol. 2010 Oct 21;55(20):6141-55. doi: 10.1088/0031-9155/55/20/007. Epub 2010 Sep 29. Phys Med Biol. 2010. PMID: 20876972 Free PMC article.
-
Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening.Phys Med Biol. 2015 Dec 7;60(23):9079-94. doi: 10.1088/0031-9155/60/23/9079. Epub 2015 Nov 12. Phys Med Biol. 2015. PMID: 26562661 Free PMC article.
-
Identifying the inertial cavitation threshold and skull effects in a vessel phantom using focused ultrasound and microbubbles.Ultrasound Med Biol. 2010 May;36(5):840-52. doi: 10.1016/j.ultrasmedbio.2010.02.009. Ultrasound Med Biol. 2010. PMID: 20420973 Free PMC article.
-
Ultrasound-induced blood-brain barrier opening.Curr Pharm Biotechnol. 2012 Jun;13(7):1332-45. doi: 10.2174/138920112800624364. Curr Pharm Biotechnol. 2012. PMID: 22201586 Free PMC article. Review.
-
Ultrasound-induced blood-brain barrier opening for drug delivery.Front Neurol Neurosci. 2015;36:106-15. doi: 10.1159/000366242. Epub 2014 Dec 22. Front Neurol Neurosci. 2015. PMID: 25531667 Review.
Cited by
-
Characterizing Focused-Ultrasound Mediated Drug Delivery to the Heterogeneous Primate Brain In Vivo with Acoustic Monitoring.Sci Rep. 2016 Nov 17;6:37094. doi: 10.1038/srep37094. Sci Rep. 2016. PMID: 27853267 Free PMC article.
-
Focused ultrasound-facilitated brain drug delivery using optimized nanodroplets: vaporization efficiency dictates large molecular delivery.Phys Med Biol. 2018 Jan 22;63(3):035002. doi: 10.1088/1361-6560/aaa30d. Phys Med Biol. 2018. PMID: 29260735 Free PMC article.
-
Contrast-Free Detection of Focused Ultrasound-Induced Blood-Brain Barrier Opening Using Diffusion Tensor Imaging.IEEE Trans Biomed Eng. 2021 Aug;68(8):2499-2508. doi: 10.1109/TBME.2020.3047575. Epub 2021 Jul 16. IEEE Trans Biomed Eng. 2021. PMID: 33360980 Free PMC article.
-
Experimental demonstration of passive acoustic imaging in the human skull cavity using CT-based aberration corrections.Med Phys. 2015 Jul;42(7):4385-400. doi: 10.1118/1.4922677. Med Phys. 2015. PMID: 26133635 Free PMC article.
-
Low-Pressure Burst-Mode Focused Ultrasound Wave Reconstruction and Mapping for Blood-Brain Barrier Opening: A Preclinical Examination.Sci Rep. 2016 Jun 13;6:27939. doi: 10.1038/srep27939. Sci Rep. 2016. PMID: 27295608 Free PMC article.
References
-
- Alexandrov AV, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004 Nov 18;351:2170–8. - PubMed
-
- Culp WC, et al. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke; a journal of cerebral circulation. 2004;35:2407–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

