MR guided thermal therapy of pancreatic tumors with endoluminal, intraluminal and interstitial catheter-based ultrasound devices: Preliminary theoretical and experimental investigations
- PMID: 24860246
- PMCID: PMC4031683
- DOI: 10.1117/12.2004669
MR guided thermal therapy of pancreatic tumors with endoluminal, intraluminal and interstitial catheter-based ultrasound devices: Preliminary theoretical and experimental investigations
Abstract
Image-guided thermal interventions have been proposed for potential palliative and curative treatments of pancreatic tumors. Catheter-based ultrasound devices offer the potential for temporal and 3D spatial control of the energy deposition profile. The objective of this study was to apply theoretical and experimental techniques to investigate the feasibility of endogastric, intraluminal and transgastric catheter-based ultrasound for MR guided thermal therapy of pancreatic tumors. The transgastric approach involves insertion of a catheter-based ultrasound applicator (array of 1.5 mm OD x 10 mm transducers, 360° or sectored 180°, ~7 MHz frequency, 13-14G cooling catheter) directly into the pancreas, either endoscopically or via image-guided percutaneous placement. An intraluminal applicator, of a more flexible but similar construct, was considered for endoscopic insertion directly into the pancreatic or biliary duct. An endoluminal approach was devised based on an ultrasound transducer assembly (tubular, planar, curvilinear) enclosed in a cooling balloon which is endoscopically positioned within the stomach or duodenum, adjacent to pancreatic targets from within the GI tract. A 3D acoustic bio-thermal model was implemented to calculate acoustic energy distributions and used a FEM solver to determine the transient temperature and thermal dose profiles in tissue during heating. These models were used to determine transducer parameters and delivery strategies and to study the feasibility of ablating 1-3 cm diameter tumors located 2-10 mm deep in the pancreas, while thermally sparing the stomach wall. Heterogeneous acoustic and thermal properties were incorporated, including approximations for tumor desmoplasia and dynamic changes during heating. A series of anatomic models based on imaging scans of representative patients were used to investigate the three approaches. Proof of concept (POC) endogastric and transgastric applicators were fabricated and experimentally evaluated in tissue mimicking phantoms, ex vivo tissue and in vivo canine model under multi-slice MR thermometry. RF micro-coils were evaluated to enable active catheter-tracking and prescription of thermometry slice positions. Interstitial and intraluminal ultrasound applicators could be used to ablate (t43>240 min) tumors measuring 2.3-3.4 cm in diameter when powered with 20-30 W/cm2 at 7 MHz for 5-10 min. Endoluminal applicators with planar and curvilinear transducers operating at 3-4 MHz could be used to treat tumors up to 20-25 mm deep from the stomach wall within 5 min. POC devices were fabricated and successfully integrated into the MRI environment with catheter tracking, real-time thermometry and closed-loop feedback control.
Keywords: MR temperature imaging; ablation; hyperthermia; modeling; pancreatic cancer; ultrasound.
Figures







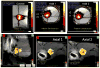
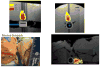

Similar articles
-
Endoluminal ultrasound applicators for MR-guided thermal ablation of pancreatic tumors: Preliminary design and evaluation in a porcine pancreas model.Med Phys. 2016 Jul;43(7):4184. doi: 10.1118/1.4953632. Med Phys. 2016. PMID: 27370138 Free PMC article.
-
Development of an endoluminal high-intensity ultrasound applicator for image-guided thermal therapy of pancreatic tumors.Proc SPIE Int Soc Opt Eng. 2015 Feb 7;9326:93260F. doi: 10.1117/12.2078841. Epub 2015 Mar 11. Proc SPIE Int Soc Opt Eng. 2015. PMID: 26677314 Free PMC article.
-
Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: in vivo evaluation under MR guidance.Med Phys. 2008 May;35(5):2081-93. doi: 10.1118/1.2900131. Med Phys. 2008. PMID: 18561684 Free PMC article.
-
Catheter-based ultrasound applicators for selective thermal ablation: progress towards MRI-guided applications in prostate.Int J Hyperthermia. 2004 Nov;20(7):739-56. doi: 10.1080/02656730410001721816. Int J Hyperthermia. 2004. PMID: 15675669 Review.
-
Magnetic resonance-guided high-intensity ultrasound ablation of the prostate.Top Magn Reson Imaging. 2006 Jun;17(3):195-207. doi: 10.1097/RMR.0b013e31803774dd. Top Magn Reson Imaging. 2006. PMID: 17414077 Review.
Cited by
-
Catheter-based ultrasound technology for image-guided thermal therapy: current technology and applications.Int J Hyperthermia. 2015 Mar;31(2):203-15. doi: 10.3109/02656736.2015.1006269. Epub 2015 Mar 23. Int J Hyperthermia. 2015. PMID: 25799287 Free PMC article. Review.
-
Magnetic Resonance-guided Active Catheter Tracking.Magn Reson Imaging Clin N Am. 2015 Nov;23(4):579-89. doi: 10.1016/j.mric.2015.05.009. Epub 2015 Jul 6. Magn Reson Imaging Clin N Am. 2015. PMID: 26499276 Free PMC article. Review.
-
Thermal therapy of pancreatic tumours using endoluminal ultrasound: Parametric and patient-specific modelling.Int J Hyperthermia. 2016;32(2):97-111. doi: 10.3109/02656736.2015.1119892. Epub 2016 Jan 21. Int J Hyperthermia. 2016. PMID: 27097663 Free PMC article.
References
-
- Bahn BM, Erdek MA. Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep. 2013;17(2):310. - PubMed
-
- Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia R, Giardino A, Orlandi M, Auriemma A, Bassi C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99(8):1083–1088. - PubMed
-
- Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD, Neugebauer A, von Renteln D, Eickhoff A, Testoni PA. Feasibility and safety of eus-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76(6):1142–1151. - PubMed
-
- Martin RC, 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: Potential improved overall survival. Ann Surg Oncol. 2012 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
