Intubating conditions and side effects of propofol, remifentanil and sevoflurane compared with propofol, remifentanil and rocuronium: a randomised, prospective, clinical trial
- PMID: 24860256
- PMCID: PMC4032635
- DOI: 10.1186/1471-2253-14-39
Intubating conditions and side effects of propofol, remifentanil and sevoflurane compared with propofol, remifentanil and rocuronium: a randomised, prospective, clinical trial
Abstract
Background: Tracheal intubation without muscle relaxants is usually performed with remifentanil and propofol or sevoflurane. Remifentanil 1.0 to 4.0 μg·kg(-1) and propofol 2.0-3.0 mg·kg(-1) or sevoflurane up to 8.0 Vol% provide acceptable, i.e. excellent or good intubating conditions. We hypothesized that sevoflurane 1.0 MAC would provide acceptable intubating conditions when combined with propofol and remifentanil.
Methods: Eighty-three patients to be intubated were randomised to two groups. The SEVO group received propofol 1.5 mg kg(-1), remifentanil 0.30 μg kg min(-1) and sevoflurane 1.0 MAC; the MR group received the same doses of propofol and remifentanil plus rocuronium 0.45 mg kg(-1). We evaluated intubation and extubation conditions, mean arterial pressure (MAP), heart rate (HR) and bispectral index (BIS). The vocal cords were examined for injury by videolaryngoscopy before and 24 hours after surgery.
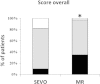
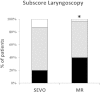
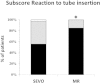
Results: ACCEPTABLE INTUBATING CONDITIONS WERE SEEN MORE FREQUENTLY WITH ROCURONIUM THAN WITH SEVOFLURANE: 97% versus 82%; p = 0.03; the subscore for vocal cords was comparable: 100% versus 98%. MAP before intubation decreased significantly compared with the MAP at baseline to the same extent in both groups; ephedrine IV was given in 15 (SEVO) versus 16 (MR) patients; p = 0.93. BIS at tracheal intubation was 27 (13-65) in the SEVO group, 29 (14-62) in the MR group; p = 0.07. Vocal cord injuries (oedema, haematoma) were similar: 4 patients in each group.
Conclusions: Overall intubating conditions were better when rocuronium was used; the subscore for vocal cords was comparable. The incidence of side effects was the same in the two groups.
Trial registration: ClinicalTrials.Gov: NCT 01591031.
Trial registration: ClinicalTrials.gov NCT01591031.
Keywords: Intubating conditions; Laryngeal injury; Neuromuscular block; Propofol; Remifentanil; Sevoflurane.
Figures

References
-
- Stevens JB, Wheatley L. Tracheal intubation in ambulatory surgery patients: using remifentanil and propofol without muscle relaxants. Anesth Analg. 1998;86:45–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

