4-[(tert-Butyl-diphenyl-sil-yloxy)meth-yl]pyridazin-3(2H)-one
- PMID: 24860296
- PMCID: PMC4004440
- DOI: 10.1107/S1600536813032212
4-[(tert-Butyl-diphenyl-sil-yloxy)meth-yl]pyridazin-3(2H)-one
Abstract
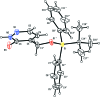
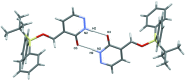
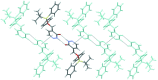
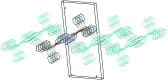
In the title compound, C21H24N2O2Si, the carbonyl group of the heterocyclic ring and the O atom of the silyl ether group are placed toward opposite sides and the tert-butyl and pyridazinone moieties are anti-oriented across the Si-O bond [torsion angle = -168.44 (19)°]. In the crystal, mol-ecules are assembled into inversion dimers through co-operative N-H⋯O hydrogen bonds between the NH groups and O atoms of the pyridazinone rings of neighbouring mol-ecules. The dimers are linked by π-π inter-actions involving adjacent pyridazinone rings [centroid-centroid distance = 3.8095 (19) Å], generating ladder-like chains along the b-axis direction. The chains are further linked into a two-dimensional network parallel to the ab plane through weak C-H⋯π inter-actions.
Figures
References
-
- Abouzid, K. & Bekhit, S. A. (2008). Bioorg. Med. Chem. 16, 5547–5556. - PubMed
-
- Al-Tel, T. H. (2010). Eur. J. Med. Chem. 45, 5724–5731. - PubMed
-
- Bruker (1998). SMART and SAINT Bruker AXS Inc., Madinson, Wisconsin, USA.
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
-
- Cesari, N., Biancanali, C., Vergelli, C., Dal Piaz, V., Graziano, A., Biagini, P., Chelardini, C., Galeotti, N. & Giovannoni, P. (2006). J. Med. Chem. 49, 7826–7835. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
