Poly[(μ4-decanedio-ato)cobalt(II)]
- PMID: 24860298
- PMCID: PMC4011297
- DOI: 10.1107/S1600536814006011
Poly[(μ4-decanedio-ato)cobalt(II)]
Abstract
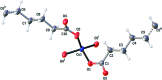
In the title compound, [Co(C10H16O4)] n , the Co(II) atom is bonded in a slightly distorted tetra-hedral environment by four O atoms from the bridging sebacate dications, comprising two separate half-ligands which lie across crystallographic inversion centres. In the three-dimensional network coordination polymer, there are two different spatial extensions of Co(II) atoms, one with the Co(II) atoms lying parallel to (100) [Co⋯Co = 4.653 (1) Å], the other lying parallel to (010) [Co⋯Co = 4.764 (1) Å].
Figures


References
-
- Biradha, K., Dennis, D., MacKinnon, V. A., Sharma, C. V. K. & Zaworotko, M. J. (1998). J. Am. Chem. Soc. 120, 11894–11903.
-
- Bond, A. D., Edwards, M. R. & Jones, W. (2001). Acta Cryst. E57, o141–o142.
-
- Borkowski, L. A. & Cahill, C. L. (2004). Acta Cryst. C60, m159–m161. - PubMed
-
- Borkowski, L. A. & Cahill, C. L. (2006). Cryst. Growth Des. 10, 2241–2247.
-
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
