{2-[(2-Bromo-5-meth-oxy-benzyl-idene)amino]-4,5,6,7-tetra-hydro-benzo[b]thiophen-3-yl}(phen-yl)methanone
- PMID: 24860382
- PMCID: PMC4011278
- DOI: 10.1107/S1600536814008290
{2-[(2-Bromo-5-meth-oxy-benzyl-idene)amino]-4,5,6,7-tetra-hydro-benzo[b]thiophen-3-yl}(phen-yl)methanone
Abstract
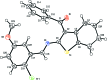
In the title compound, C23H20BrNO2S, disorder was modeled for the outer two C atoms of the cyclo-hexene ring over two sets of sites with an occupancy ratio of 0.580 (11):0.420 (11). Both rings have a half-chair conformation. The dihedral angles between the mean plane of the thio-phene ring and the benzene and phenyl rings are 9.2 (2) and 66.1 (2)°, respectively. The benzene and phenyl rings are inclined to each other by 74.8 (8)°. In the crystal, mol-ecules are linked by pairs of C-H⋯O hydrogen bonds, forming inversion dimers.
Figures
References
-
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Aydogan, F., Ocal, N., Turgut, Z. & Yolacan, C. (2001). Bull. Korean Chem. Soc. 22, 476–480.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
-
- Desai, S. B., Desai, P. B. & Desai, K. R. (2001). Hetrocycl. Commun. 7, 83–90.
LinkOut - more resources
Full Text Sources
Other Literature Sources
