Basis for a neuronal version of Grover's quantum algorithm
- PMID: 24860419
- PMCID: PMC4029008
- DOI: 10.3389/fnmol.2014.00029
Basis for a neuronal version of Grover's quantum algorithm
Abstract
Grover's quantum (search) algorithm exploits principles of quantum information theory and computation to surpass the strong Church-Turing limit governing classical computers. The algorithm initializes a search field into superposed N (eigen)states to later execute nonclassical "subroutines" involving unitary phase shifts of measured states and to produce root-rate or quadratic gain in the algorithmic time (O(N (1/2))) needed to find some "target" solution m. Akin to this fast technological search algorithm, single eukaryotic cells, such as differentiated neurons, perform natural quadratic speed-up in the search for appropriate store-operated Ca(2+) response regulation of, among other processes, protein and lipid biosynthesis, cell energetics, stress responses, cell fate and death, synaptic plasticity, and immunoprotection. Such speed-up in cellular decision making results from spatiotemporal dynamics of networked intracellular Ca(2+)-induced Ca(2+) release and the search (or signaling) velocity of Ca(2+) wave propagation. As chemical processes, such as the duration of Ca(2+) mobilization, become rate-limiting over interstore distances, Ca(2+) waves quadratically decrease interstore-travel time from slow saltatory to fast continuous gradients proportional to the square-root of the classical Ca(2+) diffusion coefficient, D (1/2), matching the computing efficiency of Grover's quantum algorithm. In this Hypothesis and Theory article, I elaborate on these traits using a fire-diffuse-fire model of store-operated cytosolic Ca(2+) signaling valid for glutamatergic neurons. Salient model features corresponding to Grover's quantum algorithm are parameterized to meet requirements for the Oracle Hadamard transform and Grover's iteration. A neuronal version of Grover's quantum algorithm figures to benefit signal coincidence detection and integration, bidirectional synaptic plasticity, and other vital cell functions by rapidly selecting, ordering, and/or counting optional response regulation choices.
Keywords: biotechnology; calcium-induced calcium reactions (CICRs); cellular decision making; classical and quantum computation; inositol 1,4,5-trisphosphate receptors (IP3Rs); intracellular calcium; neuronal plasticity; quantum molecular networks and memory.
Figures
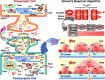
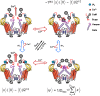
Similar articles
-
Generalized Grover's Algorithm for Multiple Phase Inversion States.Phys Rev Lett. 2018 Feb 9;120(6):060501. doi: 10.1103/PhysRevLett.120.060501. Phys Rev Lett. 2018. PMID: 29481268
-
Implementing Grover's on AES-based AEAD schemes.Sci Rep. 2024 Sep 10;14(1):21105. doi: 10.1038/s41598-024-69188-8. Sci Rep. 2024. PMID: 39256404 Free PMC article.
-
Operating Quantum States in Single Magnetic Molecules: Implementation of Grover's Quantum Algorithm.Phys Rev Lett. 2017 Nov 3;119(18):187702. doi: 10.1103/PhysRevLett.119.187702. Epub 2017 Nov 2. Phys Rev Lett. 2017. PMID: 29219608
-
Synthetic Hilbert Space Engineering of Molecular Qudits: Isotopologue Chemistry.Adv Mater. 2019 Jun;31(26):e1806687. doi: 10.1002/adma.201806687. Epub 2019 Feb 25. Adv Mater. 2019. PMID: 30803060 Review.
-
Grover's disease secondarily infected with herpes simplex virus and Staphylococcus aureus: case report and review.Australas J Dermatol. 2013 Nov;54(4):e88-91. doi: 10.1111/j.1440-0960.2012.00949.x. Epub 2012 Sep 26. Australas J Dermatol. 2013. PMID: 23013197 Review.
Cited by
-
The Osteopath's Imprint: Osteopathic Medicine Under the Nanoscopic Lens.Cureus. 2023 Jan 18;15(1):e33914. doi: 10.7759/cureus.33914. eCollection 2023 Jan. Cureus. 2023. PMID: 36660241 Free PMC article. Review.
-
Neural Field Continuum Limits and the Structure-Function Partitioning of Cognitive-Emotional Brain Networks.Biology (Basel). 2023 Feb 23;12(3):352. doi: 10.3390/biology12030352. Biology (Basel). 2023. PMID: 36979044 Free PMC article. Review.
-
Non-Local Parallel Processing and Database Settlement Using Multiple Teleportation Followed by Grover Post-Selection.Entropy (Basel). 2023 Feb 18;25(2):376. doi: 10.3390/e25020376. Entropy (Basel). 2023. PMID: 36832742 Free PMC article.
-
Fascial Manual Medicine: A Continuous Evolution.Cureus. 2024 Oct 14;16(10):e71442. doi: 10.7759/cureus.71442. eCollection 2024 Oct. Cureus. 2024. PMID: 39403420 Free PMC article. Review.
-
Quantum Computing in the Next-Generation Computational Biology Landscape: From Protein Folding to Molecular Dynamics.Mol Biotechnol. 2024 Feb;66(2):163-178. doi: 10.1007/s12033-023-00765-4. Epub 2023 May 27. Mol Biotechnol. 2024. PMID: 37244882 Free PMC article. Review.
References
-
- Adamatzky A. (2010). Physarum Machines: Computers from Slime Mould. Singapore: World Scientific Publishing Company
-
- Amos M. (2006). Genesis Machines: The New Science of Biocomputing. London: Atlantic Books
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

