Premature lethality, hyperactivity, and aberrant phosphorylation in transgenic mice expressing a constitutively active form of Fyn
- PMID: 24860422
- PMCID: PMC4026715
- DOI: 10.3389/fnmol.2014.00040
Premature lethality, hyperactivity, and aberrant phosphorylation in transgenic mice expressing a constitutively active form of Fyn
Abstract
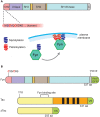
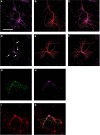
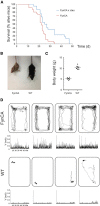
The kinase Fyn, the microtubule-associated protein tau and the peptide amyloid-β (Aβ) constitute a toxic triad in Alzheimer's disease (AD). Tau's subcellular localization is mainly regulated by phosphorylation whereas Fyn's localization is dictated by palmitoylation targeting it to the plasma membrane in a reversible manner. We have previously shown that tau is required for Fyn to be targeted to the dendritic spine. We had also shown that a truncated form of tau (Δtau) that accumulates in the cell soma is capable of trapping Fyn and preventing it from entering the spine. Here we determined that palmitoylation is required for Fyn's membrane and spine localization. We further evaluated the functional consequences of neuronal over-expression of the constitutively active Y531F mutant form of Fyn (FynCA) in transgenic mice. We found that the FynCA transgenic mice displayed a reduced weight, a massively reduced lifespan and a high level of hyperactivity. The lifespan of the FynCA mice was only slightly extended by crossing them with Δtau transgenic mice, possibly reflecting differences in expression patterns of the transgenes and high levels of transgenic FynCA compared to endogenous Fyn. Analysis of synaptosomes revealed that FynCA accumulated at high levels in the spine, resulting in increased levels of the NMDA receptor subunit NR2b phosphorylated at residue Y1472. Tau was strongly phosphorylated at the AT8 epitope S202/T205 as shown by Western blot and immunohistochemistry indicating that an increased tyrosine kinase activity of Fyn has down-stream consequences for serine/threonine-directed phosphorylation.
Keywords: Alzheimer; Fyn kinase; dendrite; palmitoylation; phosphorylation; spine; tau.
Figures


Similar articles
-
Pyk2 is a Novel Tau Tyrosine Kinase that is Regulated by the Tyrosine Kinase Fyn.J Alzheimers Dis. 2018;64(1):205-221. doi: 10.3233/JAD-180054. J Alzheimers Dis. 2018. PMID: 29782321 Free PMC article.
-
Targeting Fyn Kinase in Alzheimer's Disease.Biol Psychiatry. 2018 Feb 15;83(4):369-376. doi: 10.1016/j.biopsych.2017.06.004. Epub 2017 Jun 13. Biol Psychiatry. 2018. PMID: 28709498 Free PMC article. Review.
-
Pseudophosphorylation of Tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein Tau to dendritic spines.Biochim Biophys Acta. 2015 May;1852(5):913-24. doi: 10.1016/j.bbadis.2014.12.017. Epub 2015 Jan 3. Biochim Biophys Acta. 2015. PMID: 25558816
-
Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models.Cell. 2010 Aug 6;142(3):387-97. doi: 10.1016/j.cell.2010.06.036. Epub 2010 Jul 22. Cell. 2010. PMID: 20655099
-
Anesthesia and tau pathology.Prog Neuropsychopharmacol Biol Psychiatry. 2013 Dec 2;47:147-55. doi: 10.1016/j.pnpbp.2013.03.004. Epub 2013 Mar 25. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23535147 Free PMC article. Review.
Cited by
-
Pyk2 is a Novel Tau Tyrosine Kinase that is Regulated by the Tyrosine Kinase Fyn.J Alzheimers Dis. 2018;64(1):205-221. doi: 10.3233/JAD-180054. J Alzheimers Dis. 2018. PMID: 29782321 Free PMC article.
-
Targeting Fyn Kinase in Alzheimer's Disease.Biol Psychiatry. 2018 Feb 15;83(4):369-376. doi: 10.1016/j.biopsych.2017.06.004. Epub 2017 Jun 13. Biol Psychiatry. 2018. PMID: 28709498 Free PMC article. Review.
-
WAVE complex forms linear arrays at negative membrane curvature to instruct lamellipodia formation.J Cell Biol. 2025 Sep 1;224(9):e202410098. doi: 10.1083/jcb.202410098. Epub 2025 Jul 16. J Cell Biol. 2025. PMID: 40668190
-
Co-immunoprecipitation with Tau Isoform-specific Antibodies Reveals Distinct Protein Interactions and Highlights a Putative Role for 2N Tau in Disease.J Biol Chem. 2016 Apr 8;291(15):8173-88. doi: 10.1074/jbc.M115.641902. Epub 2016 Feb 9. J Biol Chem. 2016. PMID: 26861879 Free PMC article.
-
Fyn nanoclustering requires switching to an open conformation and is enhanced by FTLD-Tau biomolecular condensates.Mol Psychiatry. 2023 Feb;28(2):946-962. doi: 10.1038/s41380-022-01825-y. Epub 2022 Oct 18. Mol Psychiatry. 2023. PMID: 36258016 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

