Fast detection of extrasynaptic GABA with a whole-cell sniffer
- PMID: 24860433
- PMCID: PMC4030185
- DOI: 10.3389/fncel.2014.00133
Fast detection of extrasynaptic GABA with a whole-cell sniffer
Abstract
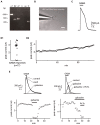
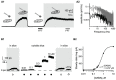
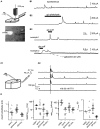
Gamma-amino-butyric acid (GABA) is the main inhibitory transmitter of the brain. It operates by binding to specific receptors located both inside and outside synapses. The extrasynaptic receptors are activated by spillover from GABAergic synapses and by ambient GABA in the extracellular space. Ambient GABA is essential for adjusting the excitability of neurons. However, due to the lack of suitable methods, little is known about its dynamics. Here we describe a new technique that allows detection of GABA transients and measurement of the steady state GABA concentration with high spatial and temporal resolution. We used a human embryonic kidney (HEK) cell line that stably expresses GABAA receptors composed of α1, β2, and γ2 subunits. We recorded from such a HEK cell with the whole-cell patch-clamp technique. The presence of GABA near the HEK cell generated a measurable electric current whose magnitude increased with concentration. A fraction of the current did not inactivate during prolonged exposition to GABA. This technique, which we refer to as a "sniffer" allows the measurement of ambient GABA concentration inside nervous tissue with a resolution of few tens of nanomolars. In addition, the sniffer detects variations in the extrasynaptic GABA concentration with millisecond time resolution. Pilot experiments demonstrate that the sniffer is able to report spillover of GABA induced by synaptic activation in real time. This is the first report on a GABA sensor that combines the ability to detect fast transients and to measure steady concentrations.
Keywords: GABA; ambient; extrasynaptic; inhibition; spillover.
Figures

Similar articles
-
Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode.Cereb Cortex. 2015 Apr;25(4):1114-23. doi: 10.1093/cercor/bht309. Epub 2013 Nov 11. Cereb Cortex. 2015. PMID: 24217990
-
γ-Aminobutyric acid type A (GABAA) receptor α subunits play a direct role in synaptic versus extrasynaptic targeting.J Biol Chem. 2012 Aug 10;287(33):27417-30. doi: 10.1074/jbc.M112.360461. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711532 Free PMC article.
-
δ subunit-containing GABAA IPSCs are driven by both synaptic and diffusional GABA in mouse dentate granule neurons.J Physiol. 2020 Mar;598(6):1205-1221. doi: 10.1113/JP279317. Epub 2020 Feb 27. J Physiol. 2020. PMID: 31951019 Free PMC article.
-
Extrasynaptic GABA(A) receptors in the brainstem and spinal cord: structure and function.Curr Pharm Des. 2013;19(24):4485-97. doi: 10.2174/1381612811319240013. Curr Pharm Des. 2013. PMID: 23360278 Review.
-
The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission.Brain Res Bull. 2013 Apr;93:69-79. doi: 10.1016/j.brainresbull.2012.08.001. Epub 2012 Aug 17. Brain Res Bull. 2013. PMID: 22925739 Review.
Cited by
-
An update of the classical and novel methods used for measuring fast neurotransmitters during normal and brain altered function.Curr Neuropharmacol. 2014 Dec;12(6):490-508. doi: 10.2174/1570159X13666141223223657. Curr Neuropharmacol. 2014. PMID: 25977677 Free PMC article.
-
Imaging with Ion Channels.Anal Chem. 2021 Apr 6;93(13):5355-5359. doi: 10.1021/acs.analchem.1c00224. Epub 2021 Mar 24. Anal Chem. 2021. PMID: 33759498 Free PMC article.
-
Exploring cells with targeted biosensors.J Gen Physiol. 2017 Jan;149(1):1-36. doi: 10.1085/jgp.201611654. Epub 2016 Dec 27. J Gen Physiol. 2017. PMID: 28028123 Free PMC article. Review.
-
Challenges of simultaneous measurements of brain extracellular GABA and glutamate in vivo using enzyme-coated microelectrode arrays.J Neurosci Methods. 2020 Jan 1;329:108435. doi: 10.1016/j.jneumeth.2019.108435. Epub 2019 Oct 7. J Neurosci Methods. 2020. PMID: 31600528 Free PMC article.
-
Diverse Actions of Astrocytes in GABAergic Signaling.Int J Mol Sci. 2019 Jun 18;20(12):2964. doi: 10.3390/ijms20122964. Int J Mol Sci. 2019. PMID: 31216630 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

