Fernando de Castro and the discovery of the arterial chemoreceptors
- PMID: 24860435
- PMCID: PMC4026738
- DOI: 10.3389/fnana.2014.00025
Fernando de Castro and the discovery of the arterial chemoreceptors
Abstract
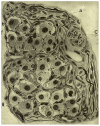
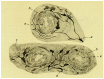
When de Castro entered the carotid body (CB) field, the organ was considered to be a small autonomic ganglion, a gland, a glomus or glomerulus, or a paraganglion. In his 1928 paper, de Castro concluded: "In sum, the Glomus caroticum is innervated by centripetal fibers, whose trophic centers are located in the sensory ganglia of the glossopharyngeal, and not by centrifugal [efferent] or secretomotor fibers as is the case for glands; these are precisely the facts which lead to suppose that the Glomus caroticum is a sensory organ." A few pages down, de Castro wrote: "The Glomus represents an organ with multiple receptors furnished with specialized receptor cells like those of other sensory organs [taste buds?]…As a plausible hypothesis we propose that the Glomus caroticum represents a sensory organ, at present the only one in its kind, dedicated to capture certain qualitative variations in the composition of blood, a function that, possibly by a reflex mechanism would have an effect on the functional activity of other organs… Therefore, the sensory fiber would not be directly stimulated by blood, but via the intermediation of the epithelial cells of the organ, which, as their structure suggests, possess a secretory function which would participate in the stimulation of the centripetal fibers." In our article we will recreate the experiments that allowed Fernando de Castro to reach this first conclusion. Also, we will scrutinize the natural endowments and the scientific knowledge that drove de Castro to make the triple hypotheses: the CB as chemoreceptor (variations in blood composition), as a secondary sensory receptor which functioning involves a chemical synapse, and as a center, origin of systemic reflexes. After a brief account of the systemic reflex effects resulting from the CB stimulation, we will complete our article with a general view of the cellular-molecular mechanisms currently thought to be involved in the functioning of this arterial chemoreceptor.
Keywords: Fernando de Castro; arterial chemoreceptorss; carotid body; ion channels; sensory physiology; transduction cascade.
Figures





References
-
- Adams W. E. (1958). The Comparative Morphology of the CB and Carotid Simus. Springfield, IL: Charles C. Thomas Publisher
-
- Almaraz L., Wang Z. Z., Dinger B., Fidone S. (1997). “Neurotransmitter mediation of carotid chemoreceptor efferent inhibition,” in The Carotid Body Chemoreceptors ed.Gonzalez C. (New York: Springer-Verlag; ) 47–158
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

