Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain
- PMID: 24860437
- PMCID: PMC4026746
- DOI: 10.3389/fnsys.2014.00038
Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain
Abstract
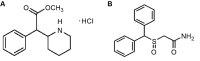
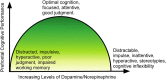
Cognitive enhancement is perhaps one of the most intriguing and controversial topics in neuroscience today. Currently, the main classes of drugs used as potential cognitive enhancers include psychostimulants (methylphenidate (MPH), amphetamine), but wakefulness-promoting agents (modafinil) and glutamate activators (ampakine) are also frequently used. Pharmacologically, substances that enhance the components of the memory/learning circuits-dopamine, glutamate (neuronal excitation), and/or norepinephrine-stand to improve brain function in healthy individuals beyond their baseline functioning. In particular, non-medical use of prescription stimulants such as MPH and illicit use of psychostimulants for cognitive enhancement have seen a recent rise among teens and young adults in schools and college campuses. However, this enhancement likely comes with a neuronal, as well as ethical, cost. Altering glutamate function via the use of psychostimulants may impair behavioral flexibility, leading to the development and/or potentiation of addictive behaviors. Furthermore, dopamine and norepinephrine do not display linear effects; instead, their modulation of cognitive and neuronal function maps on an inverted-U curve. Healthy individuals run the risk of pushing themselves beyond optimal levels into hyperdopaminergic and hypernoradrenergic states, thus vitiating the very behaviors they are striving to improve. Finally, recent studies have begun to highlight potential damaging effects of stimulant exposure in healthy juveniles. This review explains how the main classes of cognitive enhancing drugs affect the learning and memory circuits, and highlights the potential risks and concerns in healthy individuals, particularly juveniles and adolescents. We emphasize the performance enhancement at the potential cost of brain plasticity that is associated with the neural ramifications of nootropic drugs in the healthy developing brain.
Keywords: ampakine; brain development; cognitive enhancement; methylphenidate; modafinil; synaptic plasticity.
Figures
Similar articles
-
How effective are pharmaceuticals for cognitive enhancement in healthy adults? A series of meta-analyses of cognitive performance during acute administration of modafinil, methylphenidate and D-amphetamine.Eur Neuropsychopharmacol. 2020 Sep;38:40-62. doi: 10.1016/j.euroneuro.2020.07.002. Epub 2020 Jul 21. Eur Neuropsychopharmacol. 2020. PMID: 32709551
-
Psychostimulants As Cognitive Enhancers in Adolescents: More Risk than Reward?Front Public Health. 2017 Sep 26;5:260. doi: 10.3389/fpubh.2017.00260. eCollection 2017. Front Public Health. 2017. PMID: 29034227 Free PMC article. Review.
-
In search of optimal psychoactivation: stimulants as cognitive performance enhancers.Arh Hig Rada Toksikol. 2019 Sep 1;70(3):150-159. doi: 10.2478/aiht-2019-70-3298. Arh Hig Rada Toksikol. 2019. PMID: 32597132 Review.
-
Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants.Prog Neurobiol. 2013 Jan;100:60-80. doi: 10.1016/j.pneurobio.2012.10.001. Epub 2012 Oct 17. Prog Neurobiol. 2013. PMID: 23085425 Free PMC article. Review.
-
The likelihood of cognitive enhancement.Pharmacol Biochem Behav. 2011 Aug;99(2):116-29. doi: 10.1016/j.pbb.2010.12.024. Epub 2011 Jan 6. Pharmacol Biochem Behav. 2011. PMID: 21215768 Free PMC article. Review.
Cited by
-
Enhancement for well-being is still ethically challenging.Front Syst Neurosci. 2014 Apr 30;8:72. doi: 10.3389/fnsys.2014.00072. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24817843 Free PMC article. No abstract available.
-
Pharmacological profiling of zebrafish behavior using chemical and genetic classification of sleep-wake modifiers.Front Pharmacol. 2015 Nov 3;6:257. doi: 10.3389/fphar.2015.00257. eCollection 2015. Front Pharmacol. 2015. PMID: 26578964 Free PMC article.
-
Positive modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors differentially alters spatial learning and memory in juvenile rats younger and older than three weeks.Behav Pharmacol. 2024 Apr 1;35(2-3):79-91. doi: 10.1097/FBP.0000000000000764. Epub 2024 Mar 6. Behav Pharmacol. 2024. PMID: 38451022 Free PMC article.
-
A Systematic Review of the Effect of Dietary Supplements on Cognitive Performance in Healthy Young Adults and Military Personnel.Nutrients. 2020 Feb 20;12(2):545. doi: 10.3390/nu12020545. Nutrients. 2020. PMID: 32093203 Free PMC article.
-
The Psychonauts' World of Cognitive Enhancers.Front Psychiatry. 2020 Sep 11;11:546796. doi: 10.3389/fpsyt.2020.546796. eCollection 2020. Front Psychiatry. 2020. PMID: 33024436 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

