H-ferritin ferroxidase induces cytoprotective pathways and inhibits microvascular stasis in transgenic sickle mice
- PMID: 24860503
- PMCID: PMC4029007
- DOI: 10.3389/fphar.2014.00079
H-ferritin ferroxidase induces cytoprotective pathways and inhibits microvascular stasis in transgenic sickle mice
Abstract
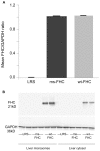
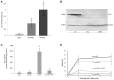
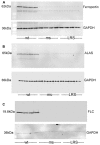
Hemolysis, oxidative stress, inflammation, vaso-occlusion, and organ infarction are hallmarks of sickle cell disease (SCD). We have previously shown that increases in heme oxygenase-1 (HO-1) activity detoxify heme and inhibit vaso-occlusion in transgenic mouse models of SCD. HO-1 releases Fe(2+) from heme, and the ferritin heavy chain (FHC) ferroxidase oxidizes Fe(2+) to catalytically inactive Fe(3+) inside ferritin. FHC overexpression has been shown to be cytoprotective. In this study, we hypothesized that overexpression of FHC and its ferroxidase activity will inhibit inflammation and microvascular stasis in transgenic SCD mice in response to plasma hemoglobin. We utilized a Sleeping Beauty (SB) transposase plasmid to deliver a human wild-type-ferritin heavy chain (wt-hFHC) transposable element by hydrodynamic tail vein injections into NY1DD SCD mice. Control SCD mice were infused with the same volume of lactated Ringer's solution (LRS) or a human triple missense FHC (ms-hFHC) plasmid with no ferroxidase activity. 8 weeks later, LRS-injected mice had ~40% microvascular stasis (% non-flowing venules) 1 h after infusion of stroma-free hemoglobin, while mice overexpressing wt-hFHC had only 5% stasis (p < 0.05), and ms-hFHC mice had 33% stasis suggesting vascular protection by ferroxidase active wt-hFHC. The wt-hFHC SCD mice had marked increases in splenic hFHC mRNA and hepatic hFHC protein, ferritin light chain (FLC), 5-aminolevulinic acid synthase (ALAS), heme content, ferroportin, nuclear factor erythroid 2-related factor 2 (Nrf2), and HO-1 activity and protein. There was also a decrease in hepatic activated nuclear factor-kappa B (NF-κB) phospho-p65 and vascular cell adhesion molecule-1 (VCAM-1). Inhibition of HO-1 activity with tin protoporphyrin demonstrated HO-1 was not essential for the protection by wt-hFHC. We conclude that wt-hFHC ferroxidase activity enhances cytoprotective Nrf2-regulated proteins including HO-1, thereby resulting in decreased NF-κB-activation, adhesion molecules, and microvascular stasis in transgenic SCD mice.
Keywords: H-ferritin; endothelium; inflammation; sickle cell disease; vaso-occlusion.
Figures




Similar articles
-
Hepatic Overexpression of Hemopexin Inhibits Inflammation and Vascular Stasis in Murine Models of Sickle Cell Disease.Mol Med. 2016 Sep;22:437-451. doi: 10.2119/molmed.2016.00063. Epub 2016 Jul 19. Mol Med. 2016. PMID: 27451971 Free PMC article.
-
Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease.J Mol Med (Berl). 2010 Jul;88(7):665-75. doi: 10.1007/s00109-010-0613-6. Epub 2010 Mar 23. J Mol Med (Berl). 2010. PMID: 20306336 Free PMC article.
-
Control of Oxidative Stress and Inflammation in Sickle Cell Disease with the Nrf2 Activator Dimethyl Fumarate.Antioxid Redox Signal. 2017 May 10;26(14):748-762. doi: 10.1089/ars.2015.6571. Epub 2016 Mar 30. Antioxid Redox Signal. 2017. PMID: 26914345 Free PMC article.
-
Patrolling monocytes in sickle cell hemolytic conditions.Transfus Clin Biol. 2019 May;26(2):128-129. doi: 10.1016/j.tracli.2019.02.004. Epub 2019 Feb 22. Transfus Clin Biol. 2019. PMID: 30898432 Free PMC article. Review.
-
The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease.Front Immunol. 2020 Mar 13;11:454. doi: 10.3389/fimmu.2020.00454. eCollection 2020. Front Immunol. 2020. PMID: 32231672 Free PMC article. Review.
Cited by
-
Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease.Haematologica. 2015 Jul;100(7):870-80. doi: 10.3324/haematol.2015.124586. Epub 2015 May 1. Haematologica. 2015. PMID: 25934765 Free PMC article.
-
Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders.Front Physiol. 2015 Jun 30;6:187. doi: 10.3389/fphys.2015.00187. eCollection 2015. Front Physiol. 2015. PMID: 26175690 Free PMC article. Review.
-
Inflammation in sickle cell disease.Clin Hemorheol Microcirc. 2018;68(2-3):263-299. doi: 10.3233/CH-189012. Clin Hemorheol Microcirc. 2018. PMID: 29614637 Free PMC article.
-
Sustained treatment of sickle cell mice with haptoglobin increases HO-1 and H-ferritin expression and decreases iron deposition in the kidney without improvement in kidney function.Br J Haematol. 2016 Nov;175(4):714-723. doi: 10.1111/bjh.14280. Epub 2016 Aug 10. Br J Haematol. 2016. PMID: 27507623 Free PMC article.
-
Pathogenesis of Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: A Case Report and Review of the Literature.Int J Mol Sci. 2024 May 29;25(11):5921. doi: 10.3390/ijms25115921. Int J Mol Sci. 2024. PMID: 38892108 Free PMC article. Review.
References
-
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., et al. (1992). Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 267 18148–18153 - PubMed
-
- Balla G., Vercellotti G. M., Muller-Eberhard U., Eaton J., Jacob H. S. (1991). Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 64 648–655 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

