Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer's disease
- PMID: 24860504
- PMCID: PMC4027795
- DOI: 10.3389/fphar.2014.00089
Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer's disease
Abstract
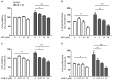
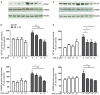
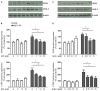
Among neurodegenerative disorders, Alzheimer's disease (AD) represents the most common cause of dementia in the elderly. Several genetic and environmental factors have been identified; however, aging represents the most important risk factor in the development of AD. To date, no effective treatments to prevent or slow this dementia are available. Sirtuins (SIRTs) are a family of NAD(+)-dependent enzymes, implicated in the control of a variety of biological processes that have the potential to modulate neurodegeneration. Here we tested the hypothesis that activation of SIRT1 or inhibition of SIRT2 would prevent reactive gliosis which is considered one of the most important hallmark of AD. Primary rat astrocytes were activated with beta amyloid 1-42 (Aβ 1-42) and treated with resveratrol (RSV) or AGK-2, a SIRT1 activator and a SIRT2-selective inhibitor, respectively. Results showed that both RSV and AGK-2 were able to reduce astrocyte activation as well as the production of pro-inflammatory mediators. These data disclose novel findings about the therapeutic potential of SIRT modulators, and suggest novel strategies for AD treatment.
Keywords: AGK-2; Alzheimer’s disease; astrocyte; beta-amyloid; reactive gliosis; resveratrol; sirtuins.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

