Retrospective and prospective perspectives on zoonotic brucellosis
- PMID: 24860561
- PMCID: PMC4026726
- DOI: 10.3389/fmicb.2014.00213
Retrospective and prospective perspectives on zoonotic brucellosis
Abstract
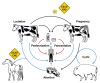
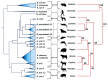
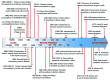
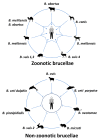
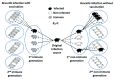
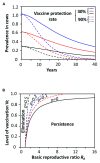
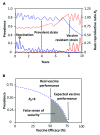
Members of the genus Brucella are pathogenic bacteria exceedingly well adapted to their hosts. The bacterium is transmitted by direct contact within the same host species or accidentally to secondary hosts, such as humans. Human brucellosis is strongly linked to the management of domesticated animals and ingestion of their products. Since the domestication of ungulates and dogs in the Fertile Crescent and Asia in 12000 and 33000 ya, respectively, a steady supply of well adapted emergent Brucella pathogens causing zoonotic disease has been provided. Likewise, anthropogenic modification of wild life may have also impacted host susceptibility and Brucella selection. Domestication and human influence on wild life animals are not neutral phenomena. Consequently, Brucella organisms have followed their hosts' fate and have been selected under conditions that favor high transmission rate. The "arm race" between Brucella and their preferred hosts has been driven by genetic adaptation of the bacterium confronted with the evolving immune defenses of the host. Management conditions, such as clustering, selection, culling, and vaccination of Brucella preferred hosts have profound influences in the outcome of brucellosis and in the selection of Brucella organisms. Countries that have controlled brucellosis systematically used reliable smooth live vaccines, consistent immunization protocols, adequate diagnostic tests, broad vaccination coverage and sustained removal of the infected animals. To ignore and misuse tools and strategies already available for the control of brucellosis may promote the emergence of new Brucella variants. The unrestricted use of low-efficacy vaccines may promote a "false sense of security" and works towards selection of Brucella with higher virulence and transmission potential.
Keywords: Brucella; Brucella-vaccines; brucellosis; domestication; zoonosis.
Figures
Comment in
-
Commentary: Retrospective and prospective perspectives on zoonotic brucellosis.Front Microbiol. 2019 Aug 14;10:1859. doi: 10.3389/fmicb.2019.01859. eCollection 2019. Front Microbiol. 2019. PMID: 31474957 Free PMC article. No abstract available.
References
-
- Abdel-Maksoud M., House B., Wasfy M., Abdel-Rahman B., Pimentel G., Roushdy G., et al. (2012). In vitro antibiotic susceptibility testing of Brucella isolates from Egypt between 1999 and 2007 and evidence of probable rifampin resistance. Ann. Clin. Microbiol. Antimicrob. 11 24 10.1186/1476-0711-11-24 - DOI - PMC - PubMed
-
- Adams G., Schutta C. J. (2010). Natural resistance against brucellosis: a review. Open Vet. Sci. J. 4 61–71
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

