Combining Hydrophilic Interaction Chromatography (HILIC) and Isotope Tagging for Off-Line LC-NMR Applications in Metabolite Analysis
- PMID: 24860727
- PMCID: PMC3901292
- DOI: 10.3390/metabo3030575
Combining Hydrophilic Interaction Chromatography (HILIC) and Isotope Tagging for Off-Line LC-NMR Applications in Metabolite Analysis
Abstract
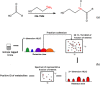
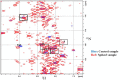
The complementary use of liquid chromatography (LC) and nuclear magnetic resonance (NMR) has shown high utility in a variety of fields. While the significant benefit of spectral simplification can be achieved for the analysis of complex samples, other limitations remain. For example, (1)H LC-NMR suffers from pH dependent chemical shift variations, especially during urine analysis, owing to the high physiological variation of urine pH. Additionally, large solvent signals from the mobile phase in LC can obscure lower intensity signals and severely limit the number of metabolites detected. These limitations, along with sample dilution, hinder the ability to make reliable chemical shift assignments. Recently, stable isotopic labeling has been used to detect quantitatively specific classes of metabolites of interest in biofluids. Here we present a strategy that explores the combined use of two-dimensional hydrophilic interaction chromatography (HILIC) and isotope tagged NMR for the unambiguous identification of carboxyl containing metabolites present in human urine. The ability to separate structurally related compounds chromatographically, in off-line mode, followed by detection using (1)H-(15)N 2D HSQC (two-dimensional heteronuclear single quantum coherence) spectroscopy, resulted in the assignment of low concentration carboxyl-containing metabolites from a library of isotope labeled compounds. The quantitative nature of this strategy is also demonstrated.
Keywords: 15N isotope tagging; HILIC; NMR; metabolite profiling; metabolomics; urine.
Figures








References
-
- Nicholson J.K., Wilson I.D. High resolution proton magnetic resonance spectroscopy of biological fluids. Prog. NMR Spectrosc. 1989;21:449–501. doi: 10.1016/0079-6565(89)80008-1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

