Navigating the rapids: the development of regulated next-generation sequencing-based clinical trial assays and companion diagnostics
- PMID: 24860780
- PMCID: PMC4029014
- DOI: 10.3389/fonc.2014.00078
Navigating the rapids: the development of regulated next-generation sequencing-based clinical trial assays and companion diagnostics
Abstract
Over the past decade, next-generation sequencing (NGS) technology has experienced meteoric growth in the aspects of platform, technology, and supporting bioinformatics development allowing its widespread and rapid uptake in research settings. More recently, NGS-based genomic data have been exploited to better understand disease development and patient characteristics that influence response to a given therapeutic intervention. Cancer, as a disease characterized by and driven by the tumor genetic landscape, is particularly amenable to NGS-based diagnostic (Dx) approaches. NGS-based technologies are particularly well suited to studying cancer disease development, progression and emergence of resistance, all key factors in the development of next-generation cancer Dxs. Yet, to achieve the promise of NGS-based patient treatment, drug developers will need to overcome a number of operational, technical, regulatory, and strategic challenges. Here, we provide a succinct overview of the state of the clinical NGS field in terms of the available clinically targeted platforms and sequencing technologies. We discuss the various operational and practical aspects of clinical NGS testing that will facilitate or limit the uptake of such assays in routine clinical care. We examine the current strategies for analytical validation and Food and Drug Administration (FDA)-approval of NGS-based assays and ongoing efforts to standardize clinical NGS and build quality control standards for the same. The rapidly evolving companion diagnostic (CDx) landscape for NGS-based assays will be reviewed, highlighting the key areas of concern and suggesting strategies to mitigate risk. The review will conclude with a series of strategic questions that face drug developers and a discussion of the likely future course of NGS-based CDx development efforts.
Keywords: clinical next-generation sequencing; companion diagnostics; disruptive technology; drug development strategy; molecular diagnostics; mutation detection methods; next-generation sequencing; precision medicine.
Figures


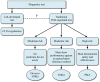
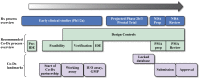
References
-
- Aftimos PG, Barthelemy P, Awada A. Molecular biology in medical oncology: diagnosis, prognosis, and precision medicine. Discov Med (2014) 17(92):81–91 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

