Long term exposure to L-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling
- PMID: 24860943
- PMCID: PMC4069264
- DOI: 10.18632/aging.100663
Long term exposure to L-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling
Abstract
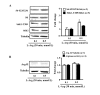
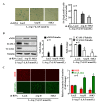
L-arginine supplementation is proposed to improve health status or as adjunct therapy for diseases including cardiovascular diseases. However, controversial results and even detrimental effects of L-arginine supplementation are reported. We investigate potential mechanisms of L-arginine-induced detrimental effects on vascular endothelial cells. Human endothelial cells were exposed to a physiological (0.1 mmol/L) or pharmacological (0.5 mmol/L) concentration of L-arginine for 30 minutes (acute) or 7 days (chronic). The effects of L-arginine supplementation on endothelial senescence phenotype, i.e., levels of senescence-associated beta-galactosidase, expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, eNOS-uncoupling, arginase-II expression/activity, and mTORC1-S6K1 activity were analyzed. While acute L-arginine treatment enhances endothelial NO production accompanied with superoxide production and activation of S6K1 but no up-regulation of arginase-II, chronic L-arginine supplementation causes endothelial senescence, up-regulation of the adhesion molecule expression, and eNOS-uncoupling (decreased NO and enhanced superoxide production), which are associated with S6K1 activation and up-regulation of arginase-II. Silencing either S6K1 or arginase-II inhibits up-regulation/activation of each other, prevents endothelial dysfunction, adhesion molecule expression, and senescence under the chronic L-arginine supplementation condition. These results demonstrate that S6K1 and arginase-II form a positive circuit mediating the detrimental effects of chronic L-arginine supplementation on endothelial cells.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





Similar articles
-
Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway.Aging (Albany NY). 2015 Jan;7(1):70-81. doi: 10.18632/aging.100722. Aging (Albany NY). 2015. PMID: 25635535 Free PMC article.
-
Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: implications for atherosclerotic plaque vulnerability.J Am Heart Assoc. 2013 Jul 5;2(4):e000096. doi: 10.1161/JAHA.113.000096. J Am Heart Assoc. 2013. PMID: 23832324 Free PMC article.
-
Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging.Aging Cell. 2012 Dec;11(6):1005-16. doi: 10.1111/acel.12001. Epub 2012 Sep 18. Aging Cell. 2012. PMID: 22928666
-
The Mechanisms of L-Arginine Metabolism Disorder in Endothelial Cells.Biochemistry (Mosc). 2021 Feb;86(2):146-155. doi: 10.1134/S0006297921020036. Biochemistry (Mosc). 2021. PMID: 33832413 Review.
-
Endothelial arginase: a new target in atherosclerosis.Curr Hypertens Rep. 2006 Apr;8(1):54-9. doi: 10.1007/s11906-006-0041-8. Curr Hypertens Rep. 2006. PMID: 16600160 Review.
Cited by
-
Immune Function of Endothelial Cells: Evolutionary Aspects, Molecular Biology and Role in Atherogenesis.Int J Mol Sci. 2022 Aug 29;23(17):9770. doi: 10.3390/ijms23179770. Int J Mol Sci. 2022. PMID: 36077168 Free PMC article. Review.
-
Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application.Int J Mol Sci. 2021 Oct 22;22(21):11427. doi: 10.3390/ijms222111427. Int J Mol Sci. 2021. PMID: 34768858 Free PMC article. Review.
-
Promotion of nitric oxide production: mechanisms, strategies, and possibilities.Front Physiol. 2025 Jan 23;16:1545044. doi: 10.3389/fphys.2025.1545044. eCollection 2025. Front Physiol. 2025. PMID: 39917079 Free PMC article. Review.
-
A nutrigeroscience approach: Dietary macronutrients and cellular senescence.Cell Metab. 2024 Sep 3;36(9):1914-1944. doi: 10.1016/j.cmet.2024.07.025. Epub 2024 Aug 22. Cell Metab. 2024. PMID: 39178854 Free PMC article. Review.
-
Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats.Nutrients. 2016 Apr 8;8(4):206. doi: 10.3390/nu8040206. Nutrients. 2016. PMID: 27070638 Free PMC article.
References
-
- Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130:2626–2629. - PubMed
-
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. - PubMed
-
- Nieves C, Jr, Langkamp-Henken B. Arginine and immunity: a unique perspective. Biomed Pharmacother. 2002;56:471–482. - PubMed
-
- McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

